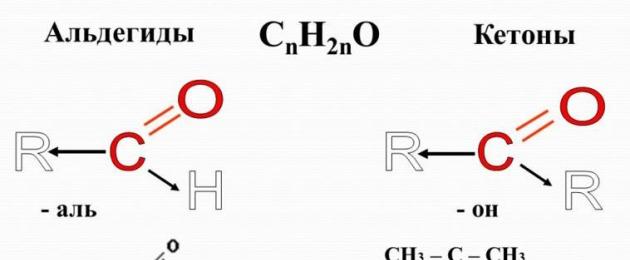
Aldehydes – organic matter, whose molecules contain a carbonyl group C=O, connected to a hydrogen atom and a hydrocarbon radical.
Aldehydes – organic matter, whose molecules contain a carbonyl group C=O, connected to a hydrogen atom and a hydrocarbon radical.
The general formula for aldehydes is:

In the simplest aldehyde, formaldehyde, the role of the hydrocarbon radical is played by another hydrogen atom:

The carbonyl group attached to the hydrogen atom is often referred to as aldehyde:
Ketones- organic substances in the molecules of which the carbonyl group is bonded to two hydrocarbon radicals. Obviously, the general formula for ketones is:

The carbonyl group of ketones is called keto group.
In the simplest ketone, acetone, the carbonyl group is bonded to two methyl radicals:

Nomenclature and isomerism of aldehydes and ketones
Depending on the structure of the hydrocarbon radical associated with the aldehyde group, limiting, unsaturated, aromatic, heterocyclic and other aldehydes are distinguished:

In accordance with the IUPAC nomenclature, the names of aldehydes are formed from the name of an alkane with the same number of carbon atoms in the molecule using the suffix -al. For example:

The numbering of carbon atoms of the main chain starts from the carbon atom of the aldehyde group. Therefore, the aldehyde group is always located at the first carbon atom, and it is not necessary to indicate its position.
Along with the systematic nomenclature, trivial names of widely used aldehydes are also used. These names are usually derived from the names of carboxylic acids corresponding to aldehydes.
For the name of ketones according to the systematic nomenclature, the keto group is denoted by the suffix -is he and a number that indicates the carbon atom number of the carbonyl group (numbering should start from the end of the chain closest to the keto group). For example:
For aldehydes, only one type of structural isomerism is characteristic - the isomerism of the carbon skeleton, which is possible from butanal, and for ketones also the isomerism of the position of the carbonyl group. In addition, they are also characterized by interclass isomerism (propanal and propanone).

Physical properties of aldehydes
In an aldehyde or ketone molecule, due to the greater electronegativity of the oxygen atom compared to the carbon atom, the bond C=O strongly polarized due to electron density shift π -bonds to oxygen:

Aldehydes and ketones are polar substances with excess electron density on the oxygen atom. The lower members of the series of aldehydes and ketones (formaldehyde, acetaldehyde, acetone) are infinitely soluble in water. Their boiling points are lower than those of the corresponding alcohols. This is due to the fact that in the molecules of aldehydes and ketones, unlike alcohols, there are no mobile hydrogen atoms and they do not form associates due to hydrogen bonds. Lower aldehydes have a pungent odor; aldehydes containing from four to six carbon atoms in the chain have an unpleasant odor; higher aldehydes and ketones have floral odors and are used in perfumery .

Chemical properties of aldehydes and ketones
The presence of an aldehyde group in a molecule determines the characteristic properties of aldehydes.
1. Recovery reactions.
The addition of hydrogen to aldehyde molecules occurs via a double bond in the carbonyl group. The product of hydrogenation of aldehydes are primary alcohols, ketones are secondary alcohols. So, when acetaldehyde is hydrogenated on a nickel catalyst, ethyl alcohol is formed, and when acetone is hydrogenated, propanol-2 is formed.

Hydrogenation of aldehydes- reduction reaction, in which the degree of oxidation of the carbon atom included in the carbonyl group decreases.
2. Oxidation reactions. Aldehydes are able not only to recover, but also oxidize. When oxidized, aldehydes form carboxylic acids.
Air oxygen oxidation. For example, propionic acid is formed from propionaldehyde (propanal):

Oxidation with weak oxidizing agents(ammonia solution of silver oxide).

If the surface of the vessel in which the reaction is carried out was previously degreased, then the silver formed during the reaction covers it with a thin, even film. It turns out a wonderful silver mirror. Therefore, this reaction is called the "silver mirror" reaction. It is widely used for making mirrors, silvering decorations and Christmas decorations.
3. Polymerization reaction:
n CH 2 \u003d O → (-CH 2 -O-) n paraforms n \u003d 8-12
Obtaining aldehydes and ketones

The use of aldehydes and ketones
Formaldehyde(methanal, formic aldehyde) H 2 C=O:
a) to obtain phenol-formaldehyde resins;
b) obtaining urea-formaldehyde (urea) resins;
c) polyoxymethylene polymers;
d) synthesis medicines(urotropin);
e) disinfectant;
f) preservative of biological preparations (due to the ability to fold the protein).
Acetic aldehyde(ethanal, acetaldehyde) CH 3 CH \u003d O:
a) production of acetic acid;
b) organic synthesis.
Acetone CH 3 -CO-CH 3:
a) solvent for varnishes, paints, cellulose acetates;
b) raw materials for the synthesis of various organic substances.
Acetic aldehyde has the chemical formula CH3COH. In appearance, it is colorless, transparent, with a pungent odor, can already boil at room temperature of 20 ° C, easily dissolves in water and organic compounds. Since science does not stand still, it is now quite simple to obtain acetaldehyde from ethyl alcohol.
The nature of the two basic substances

Acetaldehyde (ethanal) is common in nature, found in foods and in most plants. Ethanal is also a component of car exhaust and cigarette smoke, so it belongs to the category of strong toxic substances. It can be synthesized artificially in various ways. The most popular method is to obtain acetaldehyde from ethyl alcohol. Copper oxide (or silver) is used as a catalyst. The reaction produces aldehyde, hydrogen and water.
Ethyl alcohol (ethanol) is a common food grade C2H5OH. It is widely used in the manufacture of alcoholic beverages, in medicine for disinfection, in the production of household chemicals, perfumes, hygiene products and other things.
Ethyl alcohol is not found in nature, it is produced using chemical reactions. The main methods for obtaining the substance are as follows:

- Fermentation: Certain fruits or vegetables are exposed to yeast.
- Production in industrial conditions (use of sulfuric acid).
The second method gives a higher concentration of ethanol. Using the first option, it will be possible to achieve only about 16% of this substance.
Methods for obtaining acetaldehyde from ethanol
The process of obtaining acetaldehyde from ethyl alcohol occurs according to the following formula: C2H5OH + CuO = CH3CHO + Cu + H2O
In this case, ethanol and copper oxide are used, under the influence of high temperature, an oxidation reaction occurs and acetaldehyde is obtained.
There is also another method for obtaining aldehyde - dehydrogenation of alcohol. It appeared about 60 years ago and is still popular today. Dehydrogenation has many positive qualities:

- no release of toxic toxins that poison the atmosphere;
- comfortable and safe reaction conditions;
- during the reaction, hydrogen is released, which can also be used;
- no need to spend money on additional components - just one ethyl alcohol is enough.
Obtaining aldehyde by this method occurs as follows: ethanol is heated to four hundred degrees and hydrogen comes out of it in a catalytic way. The process formula looks like this: C2H5OH ͢ CH3CHO + H2.
The elimination of hydrogen occurs due to high temperature and low pressure. As soon as the temperature drops and the pressure rises, the H2 will return and the acetaldehyde will become an alcohol again.
When using the dehydration method, a copper or zinc catalyst is also used. Copper in this case is a very active substance that can lose activity during the reaction. Therefore, a mixture is made of copper, cobalt and chromium oxides, and then applied to asbestos. This makes it possible to carry out the reaction at a temperature of 270–300°C. In this case, the transformation of ethanol reaches from 34 to 50%.
Determination of the optimal method

If we compare the alcohol oxidation method with the dehydration method, then the second one has a clear advantage, since it produces much less toxic substances and at the same time the presence of a high concentration of ethanal in the contact gases is recorded. These gases, when dehydrated, contain only acetaldehyde and hydrogen, and when oxidized, they contain ethanol diluted with nitrogen. Therefore, it is easier to obtain acetaldehyde from contact gases and its losses will be much less than in the oxidation process.

Another important quality of the dehydration method is that the resulting substance is used to produce acetic acid. To do this, take mercury sulfate and water. The reaction is obtained according to the following scheme: CH3CHO + HgSO4 + H2O = CH3COOH + H2SO4 + Hg.
To complete the reaction, ferrous sulfate is added, which oxidizes the mercury. To isolate the acetic acid, the resulting solution is filtered and an alkaline solution is added.
If there is no ready-made HgSO4 (an inorganic compound from a metal salt and sulfuric acid), then it is prepared independently. It is necessary to add 1 part of mercury oxide to 4 parts of sulfuric acid.
Additional way

There is another way to obtain acetaldehyde. It is used to determine the quality of the resulting alcohol. To implement it, you will need: fuchsine sulfuric acid, ethyl alcohol and a chromium mixture (K2Cr2O7 + H2SO4).
Chromium mixture (2 ml) is poured into a dry flask, a boiling stone is placed and ethyl alcohol (2 ml) is added. The tube is covered with a tube for venting gases and the other end is inserted into a container with fuchsine sulfuric acid. The mixture is heated, as a result it changes its color to green. In the course of the reaction, ethanol is oxidized and turns into acetaldehyde, which passes through the tube in the form of vapors and, falling into a test tube with fuchsine sulphurous acid, turns it crimson.
ACETEC ALDEHYDE (acetaldehyde, ethanal) - aliphatic aldehyde, CH 3 CHO; a metabolite formed during alcoholic fermentation, oxidation of ethyl alcohol, including in the human body, and in other metabolic reactions. W. a. used in the production of various medicines (see), acetic acid (see), peracetic to-you CH 3 COOOH, acetic anhydride (CH 3 CO) 2 O, ethyl acetate, as well as in the production of synthetic resins, etc. In the relevant industries is an occupational hazard.
W. a. is a colorless liquid with a pungent odor, t° pl -123.5°, t° kip 20.2°, its relative density at 20° 0.783, refractive index at 20° 1.3316, concentration explosive limits (CEF) 3, 97 - 57%. With water, ethyl alcohol, ether, and other organic solvents U. a. mixes in any proportions.
W. a. enters into all reactions characteristic of aldehydes (see), in particular, it is oxidized to acetic acid, undergoes aldol and croton condensation, forms acetic ethyl ester according to the Tishchenko reaction and derivatives characteristic of aldehydes in the carbonyl group. In the presence of U.'s acids and. polymerizes to a cyclic crystalline tetramer of metaldehyde or liquid paraldehyde. On an industrial scale U. and. receive hydration of acetylene (see) in the presence of catalysts - salts of mercury, oxidation of ethyl alcohol (see) and the most economical way - oxidation of ethylene (see Hydrocarbons) in the presence of a palladium catalyst.
Qualitative detection U. and. is based on the appearance of blue staining as a result of the interaction of U. a. with sodium nitroprusside in the presence of amines. Quantitative definition consists in receiving any derivative At. and. by the carbonyl group and its weight, volume (see Titrimetric analysis) or colorimetric determination (see Colorimetry).
U.'s education and. as an intermediate product of metabolism occurs in both plant and animal organisms. The first stage in the conversion of ethyl alcohol in the human and animal body is its oxidation to U. a. in the presence of alcohol dehydrogenase (see). W. a. it is also formed during the decarboxylation (see) of pyruvate (see. Pyruvic acid) during alcoholic fermentation and during the breakdown of threonine (see) under the action of threonine aldolase (EC 4.1.2.5). In the human body U. a. oxidized to acetic acid Ch. arr. in the liver under the action of NAD-dependent aldehyde oxidase (EC 1.2.3. 1), acetaldehyde oxidase and xanthokinase. W. a. participates in the biosynthesis of threonine from glycine (see). In narcol. In practice, the use of those that frame (see) is based on the ability of this drug to specifically block acetaldehyde oxidase, which leads to accumulation in the blood of U. a. and, as a result, to a strong vegetative reaction - expansion of peripheral vessels, palpitations, headache, suffocation, nausea.
Acetic aldehyde as an occupational hazard
At hron. impact on the person of low concentrations of vapors U. and. note transient irritation of the mucous membranes of the upper respiratory tract and conjunctiva. Pairs of U. a. in the inhaled air in high concentrations cause increased heart rate, increased sweating; signs of a sharp irritating effect of vapors U. a. in these cases, they intensify (especially at night) and can be combined with suffocation, dry, painful cough, and headache. The consequences of such poisoning are bronchitis and pneumonia.
Contact with the skin of liquid U. a. can cause its hyperemia and the appearance of infiltrates.
First Aid and Emergency Therapy
In case of poisoning with U. pairs, a. the victim must be taken to fresh air, provide inhalation of water vapor with ammonia, if indicated - inhalation of humidified oxygen, heart remedies, respiratory stimulants (lobelin, cytoton), valerian tincture, bromine preparations. With a sharp irritation of the mucous membranes of the respiratory tract - alkaline or oil inhalations. With a painful cough - codeine, ethyl morphine hydrochloride (dionine), mustard plasters, jars. If the conjunctiva is irritated, wash the eyes with plenty of water or an isotonic solution of sodium chloride. In case of poisoning through the mouth - immediate gastric lavage with water with the addition of ammonia solution (ammonia), 3% solution of sodium bicarbonate. Further treatment is symptomatic. At hit U. and. on the skin - immediate washing of the affected area with water, but better with 5% solution of ammonia.
The victim should be removed from work with harmful substances until recovery (see Occupational diseases).
Measures for the prevention of intoxication U. a. consist in sealing of the equipment, trouble-free operation of ventilation (see), mechanization and automation of works on filling and transportation U. and. Store U. a. required in hermetically sealed containers. In industries and laboratories associated with contact with U. a., personal hygiene measures, the use of special clothing and footwear, goggles, and universal respirators must be strictly observed.
Maximum permissible concentration of vapors U. a. in the air of the working area 5 mg / m 3.
Bibliography: Harmful substances in industry, ed. N. V. Lazareva and E. N. Levina, vol. 1, L., 1976; Lebedev N. N. Chemistry and technology of basic organic and petrochemical synthesis, M., 1981; White A. et al. Fundamentals of biochemistry, trans. from English, vol. 1-3, M., 1981,
A. N. Klimov, D. V. Ioffe; N. G. Budkovskaya (giant).,
Acetic aldehyde belongs to organic compounds and belongs to the class of aldehydes. What properties does this substance have, and what does the formula of acetaldehyde look like?
general characteristics
Acetic aldehyde has several names: acetaldehyde, ethanal, methylformaldehyde. This compound is the aldehyde of acetic acid and ethanol. Its structural formula is as follows: CH 3 -CHO.

Rice. one. Chemical formula acetaldehyde.
A feature of this aldehyde is that it occurs both in nature and is produced artificially. In industry, the volume of production of this substance can be up to 1 million tons per year.
Ethanal is found in foods such as coffee, bread, and is also synthesized by plants during metabolism.
Acetic aldehyde is a colorless liquid with a pungent odor. Soluble in water, alcohol and ether. Is poisonous.

Rice. 2. Acetic aldehyde.
The liquid boils at a fairly low temperature - 20.2 degrees Celsius. Because of this, there are problems with its storage and transportation. Therefore, the substance is stored in the form of paraldehyde, and acetaldehyde is obtained from it, if necessary, by heating with sulfuric acid (or with any other mineral acid). Paraldehyde is a cyclic trimer of acetic acid.
How to get
Acetic aldehyde can be obtained in several ways. The most common option is the oxidation of ethylene or, as this method is also called, the Wacker process:
2CH 2 \u003d CH 2 + O 2 -2CH 3 CHO
The oxidizing agent in this reaction is palladium chloride.
Acetaldehyde can also be obtained by reacting acetylene with mercury salts. This reaction bears the name of a Russian scientist and is called the Kucherov reaction. As a result of the chemical process, enol is formed, which isomerizes to aldehyde
C 2 H 2 + H 2 O \u003d CH 3 CHO

Rice. 3. M. G. Kucherov portrait.
DEFINITION
Aldehydes- organic substances belonging to the class of carbonyl compounds containing in their composition the functional group -CH \u003d O, which is called carbonyl.
The general formula for limiting aldehydes and ketones is C n H 2 n O. The suffix –al is present in the name of aldehydes.
The simplest representatives of aldehydes are formaldehyde (formaldehyde) -CH 2 \u003d O, acetaldehyde (acetic aldehyde) - CH 3 -CH \u003d O. There are cyclic aldehydes, for example, cyclohexane-carbaldehyde; aromatic aldehydes have trivial names - benzaldehyde, vanillin.
The carbon atom in the carbonyl group is in a state of sp 2 hybridization and forms 3σ bonds (two C-H bonds and one C-O bond). The π-bond is formed by p-electrons of carbon and oxygen atoms. The double bond C = O is a combination of σ- and π-bonds. The electron density is shifted towards the oxygen atom.
Aldehydes are characterized by isomerism of the carbon skeleton, as well as interclass isomerism with ketones:
CH 3 -CH 2 -CH 2 -CH \u003d O (butanal);
CH 3 -CH (CH 3) -CH \u003d O (2-methylpentanal);
CH 3 -C (CH 2 -CH 3) \u003d O (methyl ethyl ketone).
Chemical properties of aldehydes
There are several reaction centers in aldehyde molecules: an electrophilic center (carbonyl carbon atom) involved in nucleophilic addition reactions; the main center is an oxygen atom with unshared electron pairs; α-CH acid center responsible for condensation reactions; S-N connection torn in oxidation reactions.
1. Addition reactions:
- water with the formation of gem-diols
R-CH \u003d O + H 2 O ↔ R-CH (OH) -OH;
- alcohols with the formation of hemiacetals
CH 3 -CH \u003d O + C 2 H 5 OH ↔CH 3 -CH (OH) -O-C 2 H 5;
- thiols with the formation of dithioacetals (in an acidic environment)
CH 3 -CH \u003d O + C 2 H 5 SH ↔ CH 3 -CH (SC 2 H 5) -SC 2 H 5 + H 2 O;
- sodium hydrosulfite with the formation of sodium α-hydroxysulfonates
C 2 H 5 -CH \u003d O + NaHSO 3 ↔ C 2 H 5 -CH (OH) -SO 3 Na;
- amines to form N-substituted imines (Schiff bases)
C 6 H 5 CH \u003d O + H 2 NC 6 H 5 ↔ C 6 H 5 CH \u003d NC 6 H 5 + H 2 O;
- hydrazines with the formation of hydrazones
CH 3 -CH \u003d O + 2 HN-NH 2 ↔ CH 3 -CH \u003d N-NH 2 + H 2 O;
- hydrocyanic acid with the formation of nitriles
CH 3 -CH \u003d O + HCN ↔ CH 3 -CH (N) -OH;
- recovery. When aldehydes react with hydrogen, primary alcohols are obtained:
R-CH \u003d O + H 2 → R-CH 2 -OH;
2. Oxidation
- the reaction of the "silver mirror" - the oxidation of aldehydes with an ammonia solution of silver oxide
R-CH \u003d O + Ag 2 O → R-CO-OH + 2Ag ↓;
- oxidation of aldehydes with copper (II) hydroxide, as a result of which a precipitate of red copper (I) oxide precipitates
CH 3 -CH \u003d O + 2Cu (OH) 2 → CH 3 -COOH + Cu 2 O ↓ + 2H 2 O;
These reactions are qualitative reactions for aldehydes.
Physical properties of aldehydes
First Representative homologous series aldehydes - formaldehyde (formaldehyde) - a gaseous substance (n.o.), aldehydes of an unbranched structure and composition C 2 -C 12 - liquids, C 13 and longer - solids. The more carbon atoms a straight-chain aldehyde contains, the higher its boiling point. With the increase molecular weight aldehydes, the values of their viscosity, density and refractive index increase. Formaldehyde and acetaldehyde are able to mix with water in unlimited quantities, however, with the growth of the hydrocarbon chain, this ability of aldehydes decreases. Lower aldehydes have a pungent odor.
Obtaining aldehydes
The main methods for obtaining aldehydes:
- hydroformylation of alkenes. This reaction consists in the addition of CO and hydrogen to an alkene in the presence of carbonyls of certain Group VIII metals, for example, octacarbonyl dicobalt (Co 2 (CO) 8) The reaction is carried out by heating to 130C and a pressure of 300 atm
CH 3 -CH \u003d CH 2 + CO + H 2 → CH 3 -CH 2 -CH 2 -CH \u003d O + (CH 3) 2 CHCH \u003d O;
— hydration of alkynes. The interaction of alkynes with water occurs in the presence of mercury (II) salts and in an acidic environment:
HC≡CH + H 2 O → CH 3 -CH \u003d O;
- oxidation of primary alcohols (the reaction proceeds when heated)
CH 3 -CH 2 -OH + CuO → CH 3 -CH \u003d O + Cu + H 2 O.
Application of aldehydes
Aldehydes have found wide application as raw materials for the synthesis of various products. So, formaldehyde (large-scale production) produces various resins (phenol-formaldehyde, etc.), drugs (urotropin); acetaldehyde is a raw material for the synthesis of acetic acid, ethanol, various pyridine derivatives, etc. Many aldehydes (butyric, cinnamon, etc.) are used as ingredients in perfumery.
Examples of problem solving
EXAMPLE 1
| The task | Bromination With n H 2 n +2 gave 9.5 g of monobromide, which, when treated with a dilute solution of NaOH, turned into an oxygen-containing compound. Its vapors with air are passed over a red-hot copper grid. When the resulting new gaseous substance was treated with an excess of an ammonia solution of Ag 2 O, 43.2 g of a precipitate was released. What hydrocarbon was taken and in what quantity, if the yield at the bromination stage is 50%, the remaining reactions proceed quantitatively. |
| Solution | We write down the equations of all occurring reactions: C n H 2n+2 + Br 2 = C n H 2n+1 Br + HBr; C n H 2n+1 Br + NaOH = C n H 2n+1 OH + NaBr; C n H 2n+1 OH → R-CH \u003d O; R-CH \u003d O + Ag 2 O → R-CO-OH + 2Ag ↓. The precipitate released in the last reaction is silver, therefore, you can find the amount of substance released silver: M(Ag) = 108 g/mol; v(Ag) \u003d m / M \u003d 43.2 / 108 \u003d 0.4 mol. According to the condition of the problem, after passing the substance obtained in reaction 2 over a hot metal grid, a gas was formed, and the only gas, aldehyde, is methanal, therefore, the initial substance is methane. CH 4 + Br 2 \u003d CH 3 Br + HBr. The amount of bromomethane substance: v (CH 3 Br) \u003d m / M \u003d 9.5/95 \u003d 0.1 mol. Then, the amount of methane substance required for a 50% yield of bromomethane is 0.2 mol. M (CH 4) \u003d 16 g / mol. Hence the mass and volume of methane: m(CH 4) = 0.2×16 = 3.2 g; V (CH 4) \u003d 0.2 × 22.4 \u003d 4.48 l. |
| Answer | Mass of methane - mass 3.2 g, volume of methane-4.48 l |
EXAMPLE 2
| The task | Write the reaction equations that can be used to carry out the following transformations: butene-1 → 1-bromobutane + NaOH → A - H 2 → B + OH → C + HCl → D. |
| Solution | To obtain 1-bromobutane from butene-1, it is necessary to carry out the hydrobromination reaction in the presence of peroxide compounds R 2 O 2 (the reaction proceeds against the Markovnikov rule): CH 3 -CH 2 -CH \u003d CH 2 + HBr → CH 3 -CH 2 -CH 2 -CH 2 Br. When interacting with an aqueous solution of alkali, 1-bromobutane undergoes hydrolysis with the formation of butanol-1 (A): CH 3 -CH 2 -CH 2 -CH 2 Br + NaOH → CH 3 -CH 2 -CH 2 -CH 2 OH + NaBr. Butanol-1 during dehydrogenation forms aldehyde - butanal (B): CH 3 -CH 2 -CH 2 -CH 2 OH → CH 3 -CH 2 -CH 2 -CH \u003d O. An ammonia solution of silver oxide oxidizes butanal to an ammonium salt - ammonium butyrate (C): CH 3 -CH 2 -CH 2 -CH \u003d O + OH →CH 3 -CH 2 -CH 2 -COONH 4 + 3NH 3 + 2Ag ↓ + H 2 O. Ammonium butyrate, when interacting with hydrochloric acid, forms butyric (butanoic) acid (D): CH 3 -CH 2 -CH 2 -COONH 4 + HCl → CH 3 -CH 2 -CH 2 -COOH + NH 4 Cl. |
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0