DEFINITION
Nickel is the twenty-eighth element of the Periodic Table. Designation - Ni from the Latin "niccolum". Located in the fourth period, VIIIB group. Refers to metals. The nuclear charge is 28.
Like cobalt, nickel occurs in nature mainly in the form of compounds with arsenic or sulfur; such, for example, are the minerals kupfernickel NiAs, the arsenic-nickel luster NiAsS, etc. Nickel is more common than cobalt [about 0.01% (mass.) of the earth's crust].
Metallic nickel has a silvery color with a yellowish tint (Fig. 1), is very hard, polishes well, and is attracted by a magnet. It is characterized by high corrosion resistance - stable in the atmosphere, in water, in alkalis and a number of acids. It actively dissolves in nitric acid. The chemical resistance of nickel is due to its tendency to passivation - to the formation of oxide films on the surface, which have a strong protective effect.
Rice. 1. Nickel. Appearance.
Atomic and molecular weight of nickel
DEFINITION
Relative molecular weight of a substance (M r) is a number showing how many times the mass of a given molecule is greater than 1/12 of the mass of a carbon atom, and relative atomic mass of an element (A r)- how many times the average mass of atoms of a chemical element is greater than 1/12 of the mass of a carbon atom.
Since nickel exists in the free state in the form of monatomic Ni molecules, the values of its atomic and molecular masses coincide. They are equal to 58.6934.
Nickel isotopes
It is known that nickel can occur in nature in the form of five stable isotopes 58Ni, 60Ni, 61Ni, 62Ni, and 64Ni. Their mass numbers are 58, 60, 61, 62 and 64, respectively. The nucleus of the nickel isotope 58 Ni contains twenty-eight protons and thirty neutrons, and the remaining isotopes differ from it only in the number of neutrons.
There are artificial unstable nickel isotopes with mass numbers from 48 to 78, as well as eight metastable states, among which the 59 Ni isotope with a half-life of 76 thousand years is the longest-lived.
nickel ions
The electronic formula showing the orbital distribution of nickel electrons is as follows:
1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2 .
As a result of chemical interaction, nickel gives up its valence electrons, i.e. is their donor, and turns into a positively charged ion:
Ni 0 -2e → Ni 2+;
Ni 0 -3e → Ni 3+.
Molecule and atom of nickel
In the free state, nickel exists in the form of monatomic Ni molecules. Here are some properties that characterize the nickel atom and molecule:
Nickel alloys
The bulk of nickel is used to produce various alloys with iron, copper, zinc, and other metals. The addition of nickel to steel increases its toughness and resistance to corrosion.
Nickel-based alloys can be divided into heat-resistant (nimonic, inconel, hastella [over 60% nickel, 15-20% chromium and other metals]), magnetic (permalloy) and alloys with special properties (monel metal, nickelin, constantan, invar, platinum).
Examples of problem solving
EXAMPLE 1
| Exercise | Write the reaction equations that can be used to carry out the following transformations: NiCl 2 → Ni → NiSO 4 → Ni(NO 3) 2 → Ni(OH) 2 → NiCl 2. Draw the equations of reactions occurring in solutions in ionic and abbreviated ionic forms. |
| Answer | By placing a nickel (II) chloride solution that is more active than nickel metal, it is possible to obtain nickel in its free form (substitution reaction): NiCl 2 + Zn → Ni + ZnCl 2 ; Ni 2+ + Zn 0 → Ni 0 + Zn 2+ . Nickel dissolves in dilute sulfuric acid to form nickel(II) sulfate: Ni + H 2 SO 4 (dilute) → NiSO 4 + H 2; Ni 0 + 2H + → Ni 2+ + H 2 . Nickel (II) nitrate can be obtained by the exchange reaction: NiSO 4 + Ba(NO 3) 2 → Ni(NO 3) 2 + BaSO 4 ↓; SO 4 2- + Ba 2+ → BaSO 4 ↓. By acting on nickel (II) nitrate with alkali, nickel (II) hydroxide can be obtained: Ni(NO 3) 2 + 2NaOH → Ni(OH) 2 ↓+ 2NaNO 3 ; Ni 2+ + 2OH - \u003d Ni (OH) 2 ↓. Nickel(II) chloride from nickel(II) hydroxide can be obtained by a neutralization reaction with hydrochloric acid: Ni(OH) 2 + 2HCl → NiCl 2 + 2H 2 O; OH - + H + \u003d H 2 O. |
EXAMPLE 2
| Exercise | What mass of nickel (II) chloride can be obtained by heating 17.7 g of nickel and 12 liters of chlorine (n.o.)? What volume of 0.06 M solution can be prepared from this mass of salt? |
| Solution | Let's write the reaction equation: Ni + Cl 2 \u003d NiCl 2. Let's find the number of moles of nickel (molar mass - 59 g / mol) and chlorine that have reacted using the data indicated in the condition of the problem: n (Ni) = m (Ni) / M (Ni); n (Ni) \u003d 17.7 / 59 \u003d 0.3 mol. n (Cl 2) \u003d V (Cl 2) / V m; n (Cl 2) \u003d 12 / 22.4 \u003d 0.54 mol. According to the equation of the problem n (Ni): n (Cl 2) = 1:1. This means that chlorine is in excess and all further calculations should be made using nickel. Let's find the amount of substance and the mass of the resulting nickel (II) chloride (molar mass 130 g / mol): n (Ni): n (NiCl 2) = 1:1; n (Ni) \u003d n (NiCl 2) \u003d 0.3 mol. m (NiCl 2)= n (NiCl 2)×M (NiCl 2); m (NiCl 2) \u003d 0.3 × 130 \u003d 39 g. Calculate the volume of a 0.06M solution that can be obtained from 39 g of nickel (II) chloride: V (NiCl 2) \u003d n (NiCl 2) / c (NiCl 2); V (NiCl 2) \u003d 0.3 / 0.06 \u003d 0.5 l. |
| Answer | The mass of nickel (II) chloride is 39 g, the volume of a 0.06M solution is 0.5 l (500 ml). |
on the topic: Nickel and its properties
The work was compiled by 2nd year students of group 5202
Nikitin Dmitry and Sharhemullin Emil.
Kazan 2013
Physical properties of Nickel.
Element was discovered in 1761. Nickel is an element of the tenth group, the fourth period of the periodic system of chemical elements. I. Mendeleev, with atomic number 28. Silver-white metal that does not tarnish in air. In its pure form, it is very plastic and can be processed by pressure. It is a ferromagnet, i.e. when conducting current through it, it has pronounced magnetic properties. Nickel atoms have an external electronic configuration 3d 8 4s 2 . It is a malleable and malleable metal, which makes it possible to produce the thinnest sheets and tubes from it.
Chemical Properties of Nickel
Chemically, Ni is similar to Fe and Co, but also to Cu and noble metals. In compounds, it exhibits variable valency (most often 2-valent). Nickel is a medium activity metal. Absorbs (especially in the finely divided state) large quantities of gases
Nickel burns only in powder form. In this case, it forms two oxides NiO and Ni 2 O 3 and, accordingly, two hydroxides Ni(OH) 2 and Ni(OH) 3 . The most important soluble nickel salts are acetate, chloride, nitrate and sulfate. Aqueous solutions of salts are usually colored green, and anhydrous salts are yellow or brown-yellow. which is often used in analytical chemistry.
N.'s saturation with gases worsens its mechanical properties. Interaction with oxygen begins at 500 °C; in a finely dispersed state, N. is pyrophoric - it ignites spontaneously in air. Of the oxides, the most important oxide is NiO - greenish crystals, practically insoluble in water (the mineral bunsenite). Hydroxide precipitates from solutions of nickel salts when alkalis are added in the form of a voluminous apple-green precipitate. When heated, H. combines with halogens, forming NiX 2 . Burning in sulfur vapor, gives a sulfide similar in composition to Ni 3 S 2 . Monosulfide NiS can be obtained by heating NiO with sulfur. N. does not react with nitrogen even at high temperatures (up to 1400 ° C)
In the liquid state, N. dissolves an appreciable amount of C, which precipitates on cooling in the form of graphite. When graphite is isolated, N. loses malleability and the ability to be processed by pressure.
Nickel is resistant to water. Organic acids act on N. only after prolonged contact with it. Sulfuric and hydrochloric acids slowly dissolve N.; dilute nitric acid - very easy; concentrated HNO 3 passivates N., but to a lesser extent than iron. When interacting with acids, salts of 2-valent Ni are formed. Almost all salts of Ni (II) and strong acids are highly soluble in water, their solutions are acidic due to hydrolysis.
Complex compounds of Nickel.
The binding of Nickel into complexes is an important diagnostic process for analytical chemistry.
Nickel is characterized by the formation of complexes. Thus, the Ni 2+ cation with ammonia forms a hexaammine complex 2+ and a diquatetraammine complex 2+ . These complexes with anions form blue or violet compounds.
Insoluble salts include oxalate and phosphate (green color), three sulfides: NiS (black), Ni 3 S 2 (yellowish-bronze) and Ni 3 S 4 (silver-white). Or, nickel dimethylglyoximate Ni (C 4 H 6 N 2 O 2) 2, giving a clear red color in an acidic environment, which is widely used in qualitative analysis for the detection of nickel.
Aqueous solutions of nickel(II) salts contain the hexaaquanickel(II) 2+ ion. When an ammonia solution is added to a solution containing these ions, nickel (II) hydroxide, a green gelatinous substance, precipitates. This precipitate dissolves when an excess amount of ammonia is added due to the formation of hexamminenickel(II) 2+ ions.
Nickel forms complexes with tetrahedral and flat square structures. For example, the tetrachloronickelate(II) 2− complex has a tetrahedral structure, while the tetracyanonickelate(II) 2− complex has a planar square structure.
The reaction of Ni 2+ ions with dimethylglyoxime is characteristic, leading to the formation of pink-red nickel dimethylglyoximate. This reaction is used in the quantitative determination of nickel, and the reaction product is used as a pigment in cosmetic materials and for other purposes.
Quantification of the Element.
It is carried out mainly by the following methods:
1) Precipitation in the form of nickel dimethoiglioximate, as already mentioned.
2) precipitation in the form of enickel-alpha-benzyldioxime.
3) Precipitation in the form of nickel hydroxide (3) . This reaction is carried out using caustic potash and bromine water.
4) Precipitation in the form of sulfide. Where nickel oxide will be used as a weight form2.
5) Electrolytic method
6) Volumetric method - i.e. titration of potassium cyanide to the formation of complex cyanide (Potassium 2 nickel ce en four times)
7) A colorimetric method based on a change in the color of the hexammine nickel ion, or the red color of a soluble complex compound, which is formed by the reaction of nickel ions 3 with dimethylglyoxime in an alkaline solution in the presence of an oxidizing agent.
8) Complexometric method.
GRAVIMETRIC METHOD FOR THE DETERMINATION OF NICKEL The method is based on the precipitation of nickel in an ammonia solution with dimethylglyoxime as a sparingly soluble intercomplex compound in the presence of citric or tartaric acid.
TITRIMETRIC METHOD FOR THE DETERMINATION OF NICKEL
The method is based on the precipitation of nickel in an ammonia solution with dimethylglyoxime as a sparingly soluble intracomplex compound in the presence of citric or tartaric acid and the determination of nickel by complexometric titration with eriochrome black T as an indicator.
Story
Nickel (English, French and German Nickel) was discovered in 1751. However, long before that, Saxon miners were well aware of the ore, which looked like copper ore and was used in glass making to color glass green. All attempts to obtain copper from this ore were unsuccessful, and therefore at the end of the 17th century. The ore was named Kupfernickel, which roughly means "Copper Devil". This ore (red nickel pyrite NiAs) was studied in 1751 by the Swedish mineralogist Kronstedt. He managed to obtain green oxide and, by reducing the latter, a new metal called nickel. When Bergman received the metal in a purer form, he found that the properties of the metal were similar to those of iron; Nickel has been studied in more detail by many chemists, beginning with Proust. Nikkel is a curse word in the language of miners. It was formed from the distorted Nicolaus, a generic word that had several meanings. But chiefly the word Nicolaus served to characterize two-faced people; in addition, it meant "a mischievous little spirit", "deceptive loafer", etc. In Russian literature of the early 19th century. the names nikolan (Scherer, 1808), nikolan (Zakharov, 1810), nicol and nickel (Dvigubsky, 1824) were used.
Physical Properties
Nickel metal has a silvery color with a yellowish tint, is very hard, ductile and malleable, polishes well, is attracted by a magnet, showing magnetic properties at temperatures below 340 ° C.
Chemical properties
Nickel dichloride (NiCl2)
Nickel atoms have an external electronic configuration of 3d84s2. The oxidation state of Ni(II) is the most stable for nickel.
Nickel forms compounds with oxidation states +2 and +3. In this case, nickel with an oxidation state of +3 is only in the form of complex salts. For nickel +2 compounds, a large number of ordinary and complex compounds are known. Nickel oxide Ni2O3 is a strong oxidizing agent.
Nickel is characterized by high corrosion resistance - it is stable in air, in water, in alkalis, in a number of acids. Chemical resistance is due to its tendency to passivation - the formation of a dense oxide film on its surface, which has a protective effect. Nickel actively dissolves in nitric acid.
With carbon monoxide CO, nickel easily forms a volatile and highly toxic carbonyl Ni(CO)4.
Finely dispersed nickel powder is pyrophoric (self-ignites in air).
Nickel burns only in powder form. Forms two oxides NiO and Ni2O3 and, respectively, two hydroxides Ni(OH)2 and Ni(OH)3. The most important soluble nickel salts are acetate, chloride, nitrate, and sulfate. Solutions are usually colored green, while anhydrous salts are yellow or brown-yellow. Insoluble salts include oxalate and phosphate (green), the three sulfides NiS (black), Ni2S3 (yellowish bronze), and Ni3S4 (black). Nickel also forms numerous coordination and complex compounds. For example, nickel dimethylglyoximate Ni(C4H6N2O2)2, which gives a clear red color in acidic media, is widely used in qualitative analysis for the detection of nickel.
An aqueous solution of nickel sulfate in a jar is green.
Aqueous solutions of nickel(II) salts contain the hexaaquanickel(II) 2+ ion. When an ammonia solution is added to a solution containing these ions, nickel (II) hydroxide, a green gelatinous substance, precipitates. This precipitate dissolves when an excess amount of ammonia is added due to the formation of hexamminenickel(II) 2+ ions.
Nickel forms complexes with tetrahedral and flat square structures. For example, the tetrachloronickelate(II) 2− complex has a tetrahedral structure, while the tetracyanonickelate(II) 2− complex has a planar square structure.
The qualitative and quantitative analysis uses an alkaline solution of butanedionedioxime, also known as dimethylglyoxime, to detect nickel(II) ions. When it interacts with nickel(II) ions, a red coordination compound bis(butanedionedioxymato)nickel(II) is formed. It is a chelate compound and the butanedionedioxymato ligand is bidentate.
Being in nature
Nickel is quite common in nature - its content in the earth's crust is approx. 0.01% (mass). It occurs in the earth's crust only in bound form; iron meteorites contain native nickel (up to 8%). Its content in ultrabasic rocks is approximately 200 times higher than in acidic ones (1.2 kg/t and 8 g/t). In ultramafic rocks, the predominant amount of nickel is associated with olivines containing 0.13–0.41% Ni. It replaces iron and magnesium isomorphically. A small part of nickel is present in the form of sulfides. Nickel exhibits siderophilic and chalcophilic properties. With an increased content of sulfur in the magma, nickel sulfides appear along with copper, cobalt, iron, and platinoids. In a hydrothermal process, together with cobalt, arsenic and sulfur, and sometimes with bismuth, uranium and silver, nickel forms elevated concentrations in the form of nickel arsenides and sulfides. Nickel is commonly found in sulfide and arsenic-bearing copper-nickel ores.
* Nickel (red nickel pyrite, kupfernickel) NiAs
* chloantite (white nickel pyrite) (Ni, Co, Fe)As2
* garnierite (Mg, Ni)6(Si4O11)(OH)6*H2O and other silicates
* magnetic pyrites (Fe, Ni, Cu)S
* arsenic-nickel gloss (gersdorfite) NiAsS,
* pentlandite (Fe,Ni)9S8
In plants, on average, 5 × 10 −5 weight percent of nickel, in marine animals - 1.6 × 10 −4, in terrestrial animals - 1 × 10 −6, in the human body - 1 ... 2 × 10 −6. Much is known about nickel in organisms. It has been established, for example, that its content in human blood changes with age, that in animals the amount of nickel in the body is increased, and finally, that there are some plants and microorganisms - "concentrators" of nickel, containing thousands and even hundreds of thousands of times more nickel than environment.
Deposits of nickel ores
The main deposits of nickel ores are located in Canada, Russia, New Caledonia, the Philippines, Indonesia, China, Finland, and Australia. Natural isotopes of nickel.
Natural nickel contains 5 stable isotopes: 58Ni (68.27%), 60Ni (26.10%), 61Ni (1.13%), 62Ni (3.59%), 64Ni (0.91%).
Receipt
The total reserves of nickel in ores at the beginning of 1998 are estimated at 135 million tons, including reliable reserves of 49 million tons.
The main ores of nickel—nickel (kupfernickel) NiAs, millerite NiS, pentlandite (FeNi)9S8—also contain arsenic, iron, and sulfur; Inclusions of pentlandite also occur in igneous pyrrhotite. Other ores from which Ni is also mined contain Co, Cu, Fe, and Mg impurities. Sometimes nickel is the main product of the refining process, but more often it is obtained as a by-product in other metal technologies. Of the reliable reserves, according to various sources, from 40 to 66% of nickel is in "oxidized nickel ores" (ONR), 33% - in sulfide, 0.7% - in others. As of 1997, the share of nickel produced by the processing of OHP was about 40% of the world's production. In industrial conditions, OHP is divided into two types: magnesian and ferruginous.
Refractory magnesian ores, as a rule, are subjected to electric smelting for ferronickel (5-50% Ni + Co, depending on the composition of the raw material and technological features).
The most ferruginous - lateritic ores are processed by hydrometallurgical methods using ammonia-carbonate leaching or sulfuric acid autoclave leaching. Depending on the composition of the raw materials and the applied technological schemes, the final products of these technologies are: nickel oxide (76-90% Ni), sinter (89% Ni), sulfide concentrates of various compositions, as well as electrolytic metal nickel, nickel powders and cobalt.
Less ferruginous - nontronite ores are melted into matte. At enterprises operating on a full cycle, a further processing scheme includes converting, roasting matte, electric smelting of nickel oxide to obtain metallic nickel. Along the way, the extracted cobalt is produced in the form of metal and/or salts. Another source of nickel: in the ashes of the coals of South Wales in England - up to 78 kg of nickel per ton. The increased content of nickel in some coals, oil, shales indicates the possibility of nickel concentration by fossil organic matter. The reasons for this phenomenon have not yet been elucidated.
The bulk of nickel is obtained from garnierite and magnetic pyrites.
1. Silicate ore is reduced with coal dust in rotary tube furnaces to iron-nickel pellets (5-8% Ni), which are then purified from sulfur, calcined and treated with an ammonia solution. After the solution is acidified, a metal is obtained electrolytically from it.
2. Carbonyl method (Mond method). First, copper-nickel matte is obtained from sulfide ore, over which CO is passed under high pressure. Easily volatile tetracarbonylnickel is formed, the thermal decomposition of which produces an especially pure metal.
3. Aluminothermic method of nickel recovery from oxide ore: 3NiO + 2Al = 3Ni + Al2O3
Application
Alloys
Nickel is the basis of most superalloys, high-temperature materials used in the aerospace industry for parts of power plants.
* Monel metal (65 - 67% Ni + 30 - 32% Cu + 1% Mn), heat resistant up to 500 °C, very corrosion resistant;
* white gold (for example, 585 samples contains 58.5% gold and an alloy (ligature) of silver and nickel (or palladium));
* nichrome, resistance alloy (60% Ni + 40% Cr);
* permalloy (76% Ni + 17% Fe + 5% Cu + 2% Cr), has a high magnetic susceptibility with very low hysteresis losses;
* Invar (65% Fe + 35% Ni), almost does not elongate when heated;
* In addition, nickel alloys include nickel and chromium-nickel steels, nickel silver, and various resistance alloys such as constantan, nickeline, and manganin.
nickel plating
Nickel plating is the creation of a nickel coating on the surface of another metal in order to protect it from corrosion. It is carried out by electroplating using electrolytes containing nickel(II) sulfate, sodium chloride, boron hydroxide, surfactants and glossy substances, and soluble nickel anodes. The thickness of the resulting nickel layer is 12–36 µm. Surface gloss stability can be ensured by subsequent chromium plating (chromium layer thickness 0.3 µm).
Currentless nickel plating is carried out in a solution of a mixture of nickel(II) chloride and sodium hypophosphite in the presence of sodium citrate:
NiCl2 + NaH2PO2 + H2O = Ni + NaH2PO3 + 2HCl
The process is carried out at pH 4-6 and 95 °C.
Battery production
Manufacture of iron-nickel, nickel-cadmium, nickel-zinc, nickel-hydrogen batteries.
Radiation technologies
The 63Ni nuclide emitting β+ particles has a half-life of 100.1 years and is used in krytrons.
Medicine
* It is used in the manufacture of bracket systems (titanium nickelide).
* Prosthetics
coinage
Nickel is widely used in the production of coins in many countries. In the United States, the 5 cent coin is colloquially known as the nickel.
Biological role
Biological role: Nickel is one of the trace elements necessary for the normal development of living organisms. However, little is known about its role in living organisms. Nickel is known to take part in enzymatic reactions in animals and plants. In animals, it accumulates in keratinized tissues, especially in feathers. An increased content of nickel in soils leads to endemic diseases - ugly forms appear in plants, and eye diseases in animals associated with the accumulation of nickel in the cornea. Toxic dose (for rats) - 50 mg. Particularly harmful are volatile nickel compounds, in particular, its tetracarbonyl Ni(CO)4. MPC of nickel compounds in air ranges from 0.0002 to 0.001 mg/m3 (for various compounds).
Physiological action
Nickel is the main cause of allergy (contact dermatitis) to metals that come into contact with the skin (jewelry, watches, jeans studs). In the European Union, the nickel content in products that come into contact with human skin is limited.
Nickel carbonyl is highly toxic. The maximum permissible concentration of its vapors in the air of industrial premises is 0.0005 mg/m³.
In the 20th century, it was found that the pancreas is very rich in nickel. When administered after insulin, nickel prolongs the action of insulin and thereby increases hypoglycemic activity. Nickel affects enzymatic processes, oxidation of ascorbic acid, accelerates the transition of sulfhydryl groups to disulfide ones. Nickel can inhibit the action of adrenaline and lower blood pressure. Excess intake of nickel in the body causes vitiligo. Nickel is deposited in the pancreas and parathyroid glands.
Nickel- a simple substance, ductile, malleable, silver-white transition metal, at ordinary temperatures in air is covered with a thin film of oxide. Chemically inactive. It belongs to heavy non-ferrous metals, it does not occur in its pure form on earth - it is usually included in various ores, has high hardness, is well polished, is a ferromagnet - is attracted by a magnet, in the periodic system of Mendeleev it is designated by the symbol Ni and has 28 serial number.
See also:
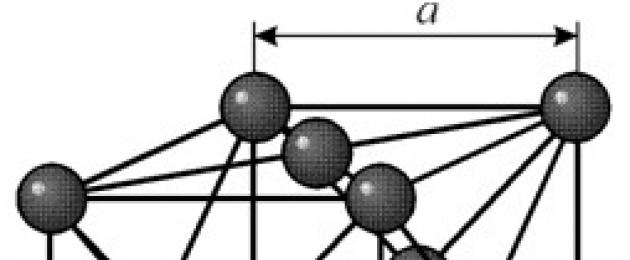
STRUCTURE
 It has a face-centered cubic lattice with a period a = 0.35238 å nm, space group Fm3m. This crystal structure is pressure resistant to at least 70 GPa. Under normal conditions, nickel exists in the form of a b-modification having a face-centered cubic lattice (a = 3.5236 å). But nickel, subjected to cathode sputtering in an atmosphere of h 2 , forms an a-modification having a hexagonal close-packed lattice (a = 2.65 å, c = 4.32 å), which, when heated above 200 °C, transforms into a cubic one. Compact cubic nickel has a density of 8.9 g / cm 3 (20 ° C), an atomic radius of 1.24 å
It has a face-centered cubic lattice with a period a = 0.35238 å nm, space group Fm3m. This crystal structure is pressure resistant to at least 70 GPa. Under normal conditions, nickel exists in the form of a b-modification having a face-centered cubic lattice (a = 3.5236 å). But nickel, subjected to cathode sputtering in an atmosphere of h 2 , forms an a-modification having a hexagonal close-packed lattice (a = 2.65 å, c = 4.32 å), which, when heated above 200 °C, transforms into a cubic one. Compact cubic nickel has a density of 8.9 g / cm 3 (20 ° C), an atomic radius of 1.24 å PROPERTIES
 Nickel is a malleable and malleable metal that can be used to make the thinnest sheets and tubes. Tensile strength 400-500 MN/m 2 , elastic limit 80 MN/m 2 , yield strength 120 MN/m 2 ; elongation 40%; modulus of normal elasticity 205 Gn/m 2 ; Brinell hardness 600-800 MN/m 2 . In the temperature range from 0 to 631K (the upper limit corresponds to the Curie point). The ferromagnetism of nickel is due to the peculiarities of the structure of the outer electron shells of its atoms. Nickel is a component of the most important magnetic materials and alloys with a minimum thermal expansion coefficient (permalloy, monel metal, invar, etc.).
Nickel is a malleable and malleable metal that can be used to make the thinnest sheets and tubes. Tensile strength 400-500 MN/m 2 , elastic limit 80 MN/m 2 , yield strength 120 MN/m 2 ; elongation 40%; modulus of normal elasticity 205 Gn/m 2 ; Brinell hardness 600-800 MN/m 2 . In the temperature range from 0 to 631K (the upper limit corresponds to the Curie point). The ferromagnetism of nickel is due to the peculiarities of the structure of the outer electron shells of its atoms. Nickel is a component of the most important magnetic materials and alloys with a minimum thermal expansion coefficient (permalloy, monel metal, invar, etc.).
RESERVES AND PRODUCTION
 Nickel is quite common in nature - its content in the earth's crust is about 0.01% (mass). It is found in the earth's crust only in bound form; iron meteorites contain native nickel (up to 8%). Its content in ultrabasic rocks is approximately 200 times higher than in acidic ones (1.2 kg/t and 8 g/t). In ultramafic rocks, the predominant amount of nickel is associated with olivines containing 0.13 - 0.41% Ni.
Nickel is quite common in nature - its content in the earth's crust is about 0.01% (mass). It is found in the earth's crust only in bound form; iron meteorites contain native nickel (up to 8%). Its content in ultrabasic rocks is approximately 200 times higher than in acidic ones (1.2 kg/t and 8 g/t). In ultramafic rocks, the predominant amount of nickel is associated with olivines containing 0.13 - 0.41% Ni.
In plants, on average, 5 10 -5 weight percent of nickel, in marine animals - 1.6 10 -4, in terrestrial animals - 1 10 -6, in the human body - 1 ... 2 10 -6.
The bulk of nickel is obtained from garnierite and magnetic pyrites.
Silicate ore is reduced with coal dust in rotary tube furnaces to iron-nickel pellets (5-8% Ni), which are then purified from sulfur, calcined and treated with an ammonia solution. After the solution is acidified, a metal is obtained electrolytically from it.
Carbonyl method (Mond method): First, copper-nickel matte is obtained from sulfide ore, over which CO is passed under high pressure. Easily volatile tetracarbonylnickel is formed, the thermal decomposition of which produces an especially pure metal.
Aluminothermic method of recovering nickel from oxide ore: 3NiO + 2Al = 3Ni + Al 2 O 3
ORIGIN
 Deposits of sulfide copper-nickel ores are associated with lopolit-like or plate-like massifs of layered gabbroids confined to zones of deep faults on ancient shields and platforms. A characteristic feature of copper-nickel deposits around the world is the consistent mineral composition of ores: pyrrhotite, pentlandite, chalcopyrite, magnetite; in addition to them, pyrite, cubanite, polydymite, nickeline, millerite, violarite, platinum group minerals, occasionally chromite, nickel and cobalt arsenides, galena, sphalerite, bornite, makinavite, wallerite, graphite, native gold are found in ores.
Deposits of sulfide copper-nickel ores are associated with lopolit-like or plate-like massifs of layered gabbroids confined to zones of deep faults on ancient shields and platforms. A characteristic feature of copper-nickel deposits around the world is the consistent mineral composition of ores: pyrrhotite, pentlandite, chalcopyrite, magnetite; in addition to them, pyrite, cubanite, polydymite, nickeline, millerite, violarite, platinum group minerals, occasionally chromite, nickel and cobalt arsenides, galena, sphalerite, bornite, makinavite, wallerite, graphite, native gold are found in ores.
Exogenous deposits of silicate nickel ores are universally associated with one or another type of weathering crust of serpentenites. during weathering, staged decomposition of minerals occurs, as well as the transfer of mobile elements, with the help of water, from the upper parts of the crust to the lower ones. There, these elements precipitate in the form of secondary minerals.
Deposits of this type contain reserves of nickel that are 3 times higher than its reserves in sulfide ores, and the reserves of some deposits reach 1 million tons or more of nickel. Large reserves of silicate ores are concentrated in New Caledonia, the Philippines, Indonesia, Australia, and other countries. The average nickel content in them is 1.1-2%. In addition, ores often contain cobalt.
APPLICATION
The vast majority of nickel is used to obtain alloys with other metals (fe, cr, cu, etc.), which are distinguished by high mechanical, anti-corrosion, magnetic or electrical and thermoelectric properties. In connection with the development of jet technology and the creation of gas turbine plants, heat-resistant and heat-resistant chromium-nickel alloys are especially important. Nickel alloys are used in the construction of nuclear reactors.
A significant amount of nickel is consumed for the production of alkaline batteries and anti-corrosion coatings. Malleable nickel in its pure form is used for the manufacture of sheets, pipes, etc. It is also used in the chemical industry for the manufacture of special chemical equipment and as a catalyst for many chemical processes. Nickel is a very scarce metal and, if possible, should be replaced by other, cheaper and more common materials.
It is used in the manufacture of bracket systems (titanium nickelide), prosthetics. It is widely used in the production of coins in many countries. In the United States, the 5 cent coin is colloquially known as the nickel. Nickel is also used for the production of winding strings of musical instruments.
Nickel - Ni
CLASSIFICATION
| Strunz (8th Edition) | 1/A.08-10 |
| Nickel-Strunz (10th edition) | 1.AA.05 |
| Dana (7th edition) | 1.1.17.2 |
| Dana (8th edition) | 1.1.11.5 | Hey's CIM Ref | 1.61 |
The properties of nickel are important parameters for finding, processing and applications of the metal. They are taken into account when forming compositions with other materials.
The properties of nickel determine its use in production
Nickel is a metal with a characteristic silvery-white color. At a temperature of 1453 °C, it becomes liquid, and boils at 2732 °C. Nickel is ductile and easily machinable under pressure.
The chemical property of nickel is characterized by its ability to form compounds with varying degrees of oxidation. Under natural conditions, a thin oxide film appears on the metal surface.
The metal has a high rate of resistance to corrosion. Nickel does not react with a number of concentrated acids and alkalis, but actively dissolves in dilute nitric acid.

Entering into chemical reactions, nickel forms volatile metals and soluble / insoluble salts
They do not react with nickel.
- inert gases;
- lithium;
- potassium;
- sodium;
- cesium;
- rubidium;
- strontium;
- barium;
- iridium;
- cesium.
Nickel combines with carbon to form carbonyl, a volatile transition metal used in the production of high purity materials. Nickel powder is capable of self-ignition upon contact with air to form oxides.
Nickel produces a number of soluble and insoluble salts. For example, a solution of metal sulfate gives the liquid a green color. Insoluble salts usually have a rich yellow color.
Forms of finding metal
Under natural conditions, nickel is found in combination with a number of chemical elements, and in the form of nuggets is found in iron meteorites.
Under hydrothermal conditions, nickel forms compounds with arsenic, cobalt, and silver. Elevated metal concentrations are associated with mineral formations - arsenides and sulfides.

In nature, nickel is usually found in compounds with other elements.
The raw material for the extraction of a valuable component is sulfide, copper-nickel ores containing arsenic:
- nickeline - a compound with arsenic;
- chloantite - white pyrites containing cobalt and iron;
- garnierite - silicate rock containing magnesium;
- magnetic pyrites - a compound of sulfur with iron and copper;
- gersdorfite - arsenic-nickel luster;
- pentlandite is a compound of sulfur, iron and nickel.
The metal content in living organisms depends on the conditions and environment. Some representatives of flora and fauna are able to concentrate the metal.
The main ore deposits are located in Canada, the Russian Federation, Albania, South Africa, Cuba, and Greece.
The process of extracting metal from ores involves the use of technologies depending on the type of raw material. Nickel is sometimes a minor enrichment material for the rock.
Refractory ores containing magnesium are subjected to electric smelting. Iron-containing lateritic ores are processed by the hydrometallurgical method, followed by treatment with alkaline solutions.
Rock with a lower iron content is melted, fired and electrically smelted. Along the way, metallic cobalt or its salts are recovered. An increased content of the metal is observed in the ash of hard coals in England. This fact is associated with the activity of microorganisms concentrating nickel.
Plasticity and other physical properties of nickel compounds depend on the purity of the material. A slight admixture of sulfur makes the metal brittle. The addition of magnesium to the molten material purifies the mixture of minor impurities with the formation of a compound with sulfur.
Industries where nickel is used
The physical and chemical properties of the metal determine its use:
- in the manufacture of stainless steel;
- for the formation of alloys that do not contain iron;
- for the purpose of applying protective coatings to products by galvanic method;
- for the production of chemical reagents;
- in powder metallurgy.
The metal is used in the manufacture of batteries, with its help catalytic processes of chemical reactions occur in industrial production. Titanium alloys are an excellent material for making dentures and tooth alignment devices.
The composition based on the chemical element No. 28 is a raw material for minting coins, manufacturing electronic cigarette coils. It is used to wind the strings of musical instruments.
In the manufacture of cores for electromagnets, compositions are used - permalloys, including 20–60% iron. Nickel is used in the manufacture of various parts and equipment for the chemical industry.
Metal oxides are used in the production of glass, glazes and ceramics. Modern production specializes in the manufacture of a variety of rolled products: wire, tape, foil, tubes.

Nickel has a wide range of applications from coatings to chemicals
Resistance to aggressive environments makes it possible to use rolled nickel for transporting alkalis in the chemical industry.
Nickel-based alloy tools are used in medicine and scientific research. The metal is used to create precision instruments for remote control of processes in the nuclear power industry, and radar installations.
Characteristics of nickel alloys
In the compositions, the metal is combined mainly with iron and cobalt. It is used as a master alloy component for the production of various structural steels, magnetic and non-magnetic alloys.
Metal alloys based on chemical element No. 28 have strength, resistance to temperatures, deformation, and environmental influences. Their number reaches several thousand. The most common compositions are combinations with chromium, molybdenum, aluminum, titanium, beryllium.
The metal is considered to be the ligature component of gold, which gives jewelry its characteristic white color and strength. In relation to this composition, there are opinions about the allergic effect of nickel on the skin.
In combination with chromium, a nichrome compound is formed, which is resistant to high temperatures, has a minimum coefficient of electrical resistance, and ductility.
It is used for the manufacture of heating devices, parts, as a coating. The high strength of the connection allows it to be machined, turned, welded, stamped.

Nickel alloys have high strength, which allows them to be widely used in production.
A special group is formed by alloys, which include copper. Among them, the most popular are:
- monel;
- brass;
- bronze;
- nickel silver.
More than a century ago, it was found that an iron-nickel composition containing 28% of the described metal loses its magnetization properties. Alloys containing 36% nickel are characterized by a slight linear expansion, which allows it to be used in the manufacture of precision instruments and tools.
This composition, which is designated FeNi36, is called invar, that is, "unchanging". Kovar alloy containing 29% nickel, 17% cobalt and 54% iron has found wide application in production.
It has high adhesion to molten glass, which allows the composition to be used for making electrical leads passing through this substance.
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0