The TCA cycle is the final stage of the catabolism of carbohydrates, lipids and proteins, during which the two-carbon acetyl residue is decomposed to 2 molecules of carbon dioxide.
1. The initial reaction is the condensation of acetyl and an oxaloacetate molecule to form citric acid (citrate)
Enzyme: citrate synthase. The rate of the reaction depends on the amount of oxaloacetate, which is both a substrate and an allosteric activator for citrate synthase.
2. Conversion of citric acid into isocitric acid (citrate into isocitrate). The reaction proceeds in two stages with the formation of an intermediate product, cis-aconitic acid.
Enzyme: aconitase. Under cellular conditions, the equilibrium in the system of these two reactions is shifted towards the formation of isocitrate, due to its constant loss in the subsequent reaction.
3. Oxidation (dehydrogenation) of isocitric acid (isocitrate). This is the first dehydrogenation reaction in the TCA cycle and serves as a potential source of energy. During this reaction, the first molecule of carbon dioxide is removed.
Enzyme : isocitrate dehydrogenase. Contains NAD+ as a coenzyme. This is the main regulatory enzyme of the cycle, its effectors: activator - NAD +, inhibitor - NADH.
Since the initial intermediate products of the process under study are tricarboxylic acids, it is called the tricarboxylic acid cycle, and according to the researcher - the Krebs cycle.
4. Oxidative decarboxylation of a-ketoglutaric acid. This is the second dehydrogenation reaction in the TCA cycle and the second reaction accompanied by the formation of the final product - CO 2. The equilibrium in this reaction is so shifted to the right that it can be considered physiologically irreversible
Enzyme: multienzyme complex a- ketoglutarate dehydrogenase. The complex includes 3 enzymes:
1. a-ketoglutarate decarboxylase
2. transacylase
3. dihydrolipoyl dehydrogenase
The complex includes 5 coenzymes: TDP, lipoic acid, NS-CoA, FAD, NAD +.
5. Reaction of III substrate phosphorylation
This reaction is associated with the formation of ATP.
Enzyme: succinate thiokinase.
Substrate phosphorylation This is a method of synthesizing ATP or GTP using the energy of high-energy molecules. The biological role of the process is the rapid production of ATP in the cell without the consumption of oxygen.
6. Oxidation of succinic acid (succinate). 3rd dehydrogenation reaction.
Enzyme:succinate dehydrogenase. Contains FAD as a coenzyme. This is the only TCA cycle enzyme that is not located in the soluble part of the matrix, but is associated with the inner mitochondrial membrane. Malonic acid, a structural analogue of succinic acid, can be used as a competitive inhibitor of this enzyme.
7. Formation of malic acid (malate)
Enzyme: fumarase. This enzyme has stereochemical specificity and is capable of adding water at the double bond only in the trans conformation.
8. Oxidation of malic acid (malate) – 4th dehydrogenation reaction.
Enzyme: malate dehydrogenase. Contains NAD+ as a coenzyme.
The oxaloacetate formed during the reactions is also the initial substrate, which makes the process cyclic.
Biological role of the Krebs cycle :
The TCA cycle is the central metabolic pathway that is associated with the transformation of all other classes of biomolecules. Performs two main functions
1. energy function. The TCA cycle is the main supplier of hydrogens in the composition of NADH and FADH 2 to the respiratory chain. Subsequently, the e contained in these hydrogens are transferred with the participation of enzymes of the respiratory chain to oxygen with the formation of the final oxidation product - water, and the energy released in this case is used for the synthesis of ATP. The TCA cycle is an aerobic process that requires constant oxygen. In the absence of oxygen, the accumulation of reduced forms of NADH and FADH occurs and, as a result, inhibition of dehydrogenation reactions of the TCA cycle occurs.
In addition, during TCA cycle reactions, 1 mol of GTP is formed in the substrate phosphorylation reaction.
2. Amphibolic function.
Under amphibolic function of the Krebs cycle understand the use of intermediates (intermediate products) of the cycle for the synthesis of other molecules. For example, succinyl-CoA is the starting compound in heme synthesis; a-ketoglutarate – amino acids (glutamate, glutamine, proline, histidine).
The use of intermediate products of the Krebs cycle for synthetic processes leads to a decrease in the level of oxaloacetate in mitochondria, inhibition of the cycle and disruption of energy metabolism. To prevent this from happening, there are reactions in the mitochondria that replenish the oxaloacetate pool.
Reactions that replenish the supply of oxaloacetate in the mitochondria are called anaplerotic.
1. Carboxylation of pyruvate:
Enzyme:pyruvate carboxylase
2. Transamination of aspartic acid:
Aspartate + a-KG oxaloacetate + glutamate
Enzyme: aspartate aminotransferase.
Regulation of the Krebs cycle.
Regulation is carried out through two mechanisms:
1. Phosphorylation-dephosphorylation. With a high level of ATP in mitochondria, phosphorylation of the 1st enzyme occurs - citrate synthase and the reaction rate of the Krebsya cycle decreases. With a decrease in ATP and accumulation of ADP, the enzyme is dephosphorylated and its activity increases.
2. Allosteric regulation. This mechanism is used to regulate two enzymes.
Citrate synthase activated by oxaloacetate.
Isocitrate dehydrogenase(major regulatory enzyme) is activated by NAD + and inhibited by NADH 2
a-ketoglutarate dehydrogenase inhibited by the reaction product - succinyl-CoA.
Tricarboxylic acid cycle (CTK) or citric acid cycle or Krebs cycle – the path of oxidative transformations of di- and tricarboxylic acids formed as intermediate products during the breakdown and synthesis of proteins, fats and carbohydrates.
The tricarboxylic acid cycle is present in the cells of all organisms: plants, animals and microorganisms.
This cycle is basis of metabolism and executes two important functions:
- supplying the body with energy ;
- integration of all major metabolic pathways, both catabolic (biodegradation) and anabolic (biosynthesis) .
Let me remind you that the reactions of aerobic glycolysis localized in cytoplasm cells and lead to the formation pyruvate (PVK).
!!! Subsequent transformations pyruvate flow into mitochondrial matrix.
In the matrix, pyruvate is converted to acetyl-CoA – high energy compound. The reaction is catalyzed by an enzyme NAD-dependent pyruvate decarboxylase:
Restored form NADH∙H + , formed as a result of this reaction enters respiratory chain and generates 6 ATP molecules(calculated per 1 molecule of glucose).
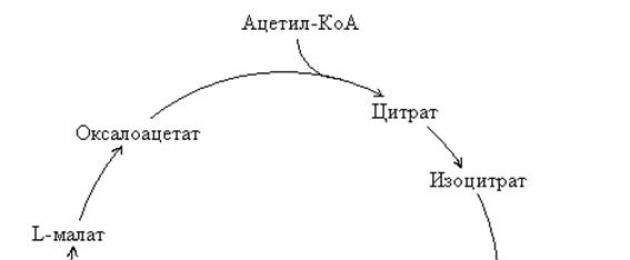
!!! The TCA cycle is a sequence of eight reactions occurring in the matrix mitochondria (Rice. 9.6):
Rice. 9.6. Diagram of the tricarboxylic acid cycle
1) Irreversible reaction condensation acetyl-CoA with oxaloacetic acid (oxaloacetate), catalyzed by an enzyme citrate synthetase, with education citric acid (citrate ).
2) Reversible reaction isomerization citric acid (citrate ) V isocitric acid (isocitrate ), during which occurs transfer of a hydroxy group to another carbon atom, catalyzed by the enzyme aconitase .
The reaction comes through education intermediate product
cis-acanitic acid (cis-aconitate
).
3) Irreversible reaction oxidative decarboxylation isocitric acid
(isocitrate
): hydroxy group isocitric acid oxidizes to a carbonyl group using the oxidized form NAD +
and at the same time carboxyl group is removed from
β-position with education α-ketoglutaric acid
(α-ketoglutarate
). The intermediate product of this reaction oxalosuccinic acid
(oxalosuccinate
).
This is the first reaction of the cycle, in which the oxidized form of NAD + coenzyme is reduced to NADH∙H +, the enzyme isocitrate dehydrogenase.
Restored form NADH∙H enters respiratory chain, there it oxidizes to NAD +, which leads to the formation 2 molecules ATP .
4) Reversible reaction oxidative decarboxylation
α-ketoglutaric acid
before macroergic connections succinyl-CoA
. The reaction is catalyzed by an enzyme 2-oxoglutarate dehydrogenase complex.
5) Reaction is the only reaction in the cycle; catalyzed by enzyme succinyl-CoA synthetase. In this reaction succinyl-CoA starring guanodine diphosphate (GDF ) And inorganic phosphate (H3PO4 ) turns into succinic acid (succinate ).
!!! At the same time, the synthesis of the high-energy compound GTP occurs due to macroergic connection thioether bond succinyl-CoA.
6) Reaction dehydrogenation succinic acid (succinate ) with education fumaric acid (fumarate).
The reaction is catalyzed by a complex enzyme succinate dehydrogenase, in the molecule of which the coenzyme FAD + covalently bound, but by the protein part of the enzyme. Oxidized form FAD + as a result of the reaction it is reduced to FAD∙N 2.
Restored form FAD∙N 2 enters respiratory chain, there it regenerates to the oxidized form FAD +, which leads to the formation two molecules ATP.
7) Reaction hydration fumaric acid (fumarate ) before malic acid (malate fumarase.
8) Reaction dehydrogenation malic acid before oxaloacetic acid (oxaloacetate ). The reaction is catalyzed by an enzyme NAD+-dependent-malate dehydrogenase.
As a result of the reaction, the oxidized form NAD is restored to restored form NADH∙H +.
Restored form NADH∙H enters respiratory chain, there it oxidizes to NAD +, which leads to the formation 2 ATP molecules.
Summary equation The CTC can be written as follows:
Acetyl-CoA + 3NAD + + FAD + + GDP + H 3 PO 4 =
2CO2 + H2O + HS-CoA + 3NADH∙H + FAD∙H2 + GTP
As can be seen from the diagram of the total equation of the TTC in this process, the following are restored:
Three molecules NADH∙H(reactions 3, 4, 8);
One molecule FAD∙N 2(reaction 6).
During aerobic oxidation, from these molecules in the electron transport chain in the process of oxidative phosphorylation, the following is formed during oxidation:
- one molecules NADH∙H – 3 molecules ATP ;
- one molecules FAD∙N 2 – 2 molecules ATP.
- one molecule GTF formed in the reaction substrate phosphorylation (reaction 5).
All this will amount to: 9 (3x3) ATP + 2 ATP + 1 ATP (GTF ) = 12 ATP . Hence, energy balance oxidation acetyl-CoA (2 molecules pyruvate from aerobic glycolysis) V CTK amounts to 24 molecules ATP .
!!! Complete oxidation glucose :
8 molecules ATP
glycolysis + 6 molecules ATP
oxidative decarboxylation of pyruvate cetyl-CoA
+ 24 molecules ATP
CTK =
38 molecules ATP
per glucose molecule.
The tricarboxylic acid cycle was discovered in 1937 by G. Krebs. In this regard, it was called the “Krebs cycle”. This process is the central pathway of metabolism. It occurs in the cells of organisms at different stages of evolutionary development (microorganisms, plants, animals).
The initial substrate of the tricarboxylic acid cycle is acetyl coenzyme A. This metabolite is the active form of acetic acid. Acetic acid acts as a common intermediate breakdown product of almost all organic substances contained in the cells of living organisms. This is because organic molecules are carbon compounds that can naturally break down into two-carbon acetic acid units.
Free acetic acid has a relatively weak reactivity. Its transformations occur under rather harsh conditions, which are unrealistic in a living cell. Therefore, acetic acid is activated in cells by combining it with coenzyme A. As a result, a metabolically active form of acetic acid is formed - acetyl-coenzyme A.
Coenzyme A is a low molecular weight compound that consists of phosphoadenosine, a pantothenic acid residue (vitamin B3) and thioethanolamine. The acetic acid residue is added to the sulfhydryl group of thioethanolamine. In this case, a thioether is formed - acetyl-coenzyme A, which is the initial substrate of the Krebs cycle.
Acetyl coenzyme A
A diagram of the transformation of intermediate products in the Krebs cycle is shown in Fig. 67. The process begins with the condensation of acetyl coenzyme A with oxaloacetate (oxaloacetic acid, OCA), resulting in the formation of citric acid (citrate). The reaction is catalyzed by the enzyme citrate synthase.

Figure 67 – Scheme of the transformation of intermediate products in the cycle
tricarboxylic acids
Further, under the action of the enzyme aconitase, citric acid is converted into isocitric acid. Isocitric acid undergoes oxidation and decarboxylation processes. In this reaction, catalyzed by the enzyme NAD-dependent isocitrate dehydrogenase, the products are carbon dioxide, reduced NAD, and a-ketoglutaric acid, which is then involved in the process of oxidative decarboxylation (Fig. 68).


Figure 68 – Formation of a-ketoglutaric acid in the Krebs cycle
The process of oxidative decarboxylation of a-ketoglutarate is catalyzed by the enzymes of the a-ketoglutarate dehydrogenase multienzyme complex. This complex consists of three different enzymes. It requires coenzymes to function. Coenzymes of the a-keto-glutarate dehydrogenase complex include the following water-soluble vitamins:
· vitamin B 1 (thiamine) – thiamine pyrophosphate;
· vitamin B 2 (riboflavin) – FAD;
· vitamin B 3 (pantothenic acid) – coenzyme A;
· vitamin B 5 (nicotinamide) – NAD;
· vitamin-like substance – lipoic acid.
Schematically, the process of oxidative decarboxylation of a-keto-glutaric acid can be represented as the following balance reaction equation:

The product of this process is a thioester of the succinic acid residue (succinate) with coenzyme A - succinyl-coenzyme A. The thioester bond of succinyl-coenzyme A is macroergic.
The next reaction of the Krebs cycle is the process of substrate phosphorylation. In it, the thioester bond of succinyl-coenzyme A is hydrolyzed under the action of the enzyme succinyl-CoA synthetase with the formation of succinic acid (succinate) and free coenzyme A. This process is accompanied by the release of energy, which is immediately used for phosphorylation of HDP, which results in the formation of a high-energy molecule GTP phosphate. Substrate phosphorylation in the Krebs cycle:

where Fn is orthophosphoric acid.
GTP formed during oxidative phosphorylation can be used as an energy source in various energy-dependent reactions (in the process of protein biosynthesis, activation of fatty acids, etc.). In addition, GTP can be used to generate ATP in the nucleoside diphosphate kinase reaction
The product of the succinyl-CoA synthetase reaction, succinate, is further oxidized with the participation of the enzyme succinate dehydrogenase. This enzyme is a flavin dehydrogenase, which contains the FAD molecule as a coenzyme (prosthetic group). As a result of the reaction, succinic acid is oxidized to fumaric acid. At the same time, FAD is restored.

where E is the FAD prosthetic group associated with the polypeptide chain of the enzyme.
Fumaric acid formed in the succinate dehydrogenase reaction, under the action of the fumarase enzyme (Fig. 69), attaches a water molecule and is converted into malic acid, which is then oxidized in the malate dehydrogenase reaction into oxaloacetic acid (oxaloacetate). The latter can be used again in the citrate synthase reaction for the synthesis of citric acid (Fig. 67). Due to this, transformations in the Krebs cycle are cyclic in nature.

Figure 69 – Metabolism of malic acid in the Krebs cycle
The balance equation of the Krebs cycle can be presented as:
It shows that in the cycle there is complete oxidation of the acetyl radical of the residue from acetyl-coenzyme A to two molecules of CO 2. This process is accompanied by the formation of three molecules of reduced NAD, one molecule of reduced FAD and one molecule of high-energy phosphate - GTP.
The Krebs cycle occurs in the mitochondrial matrix. This is due to the fact that this is where most of its enzymes are located. And only a single enzyme, succinate dehydrogenase, is built into the inner mitochondrial membrane. The individual enzymes of the tricarboxylic acid cycle are combined into a functional multienzyme complex (metabolon) associated with the inner surface of the inner mitochondrial membrane. By combining enzymes into a metabolon, the efficiency of functioning of this metabolic pathway is significantly increased and additional opportunities for its fine regulation appear.
Features of the regulation of the tricarboxylic acid cycle are largely determined by its significance. This process performs the following functions:
1) energy. The Krebs cycle is the most powerful source of substrates (reduced coenzymes - NAD and FAD) for tissue respiration. In addition, energy is stored in it in the form of high-energy phosphate - GTP;
2) plastic. Intermediate products of the Krebs cycle are precursors for the synthesis of various classes of organic substances - amino acids, monosaccharides, fatty acids, etc.
Thus, the Krebs cycle performs a dual function: on the one hand, it is a general pathway of catabolism, playing a central role in the energy supply of the cell, and on the other, it provides biosynthetic processes with substrates. Such metabolic processes are called amphibolic. The Krebs cycle is a typical amphibolic cycle.
The regulation of metabolic processes in the cell is closely related to the existence of “key” enzymes. The key enzymes in the process are those that determine its speed. Typically, one of the “key” enzymes in a process is the enzyme that catalyzes its initial reaction.
The “key” enzymes are characterized by the following features. These enzymes
· catalyze irreversible reactions;
· have the least activity compared to other enzymes involved in the process;
· are allosteric enzymes.
The key enzymes of the Krebs cycle are citrate synthase and isocitrate dehydrogenase. Like key enzymes in other metabolic pathways, their activity is regulated by negative feedback: it decreases as the concentration of Krebs cycle intermediates in mitochondria increases. Thus, citric acid and succinyl-coenzyme A act as citrate synthase inhibitors, and reduced NAD acts as isocitrate dehydrogenase.
ADP is an activator of isocitrate dehydrogenase. Under conditions of increasing cell need for ATP as an energy source, when the content of breakdown products (ADP) increases in it, prerequisites arise for increasing the rate of redox transformations in the Krebs cycle and, consequently, increasing the level of its energy supply.
In the 30s of the twentieth century, the German scientist Hans Krebs, together with his student, studied the circulation of urea. During World War II, Krebs moved to England where he came to the conclusion that certain acids catalyze processes in our body. For this discovery he was awarded the Nobel Prize.
As you know, the energy potential of the body depends on the glucose contained in our blood. Also, the cells of the human body contain mitochondria, which help in processing glucose to convert it into energy. After some transformations, glucose is converted into a substance called adenosine triphosphate (ATP), the main source of energy for cells. Its structure is such that it can be incorporated into a protein, and this compound will provide energy to all human organ systems. Glucose cannot directly become ATP, so complex mechanisms are used to obtain the desired result. This is the Krebs cycle.
In very simple terms, the Krebs cycle is a chain of chemical reactions occurring in every cell of our body, which is called a cycle because it continues continuously. The end result of this cycle of reactions is the production of adenosine triphosphate, a substance that represents the energy basis of the body's functioning. This cycle is otherwise called cellular respiration, since most of its stages occur with the participation of oxygen. In addition, the most important function of the Krebs cycle is distinguished - plastic (construction), since during the cycle elements important for life are produced: carbohydrates, amino acids, etc.
To implement all of the above, it is necessary to have more than a hundred different elements, including vitamins. If at least one of them is absent or deficient, the cycle will not be efficient enough, which will lead to metabolic disorders throughout the human body.
Stages of the Krebs cycle
- The first step is the splitting of glucose molecules into two molecules of pyruvic acid. Pyruvic acid performs an important metabolic function; liver function directly depends on its action. It has been proven that this compound is found in some fruits, berries and even honey; it is successfully used in cosmetology as a way to combat dead epithelial cells (gommage). Also, as a result of the reaction, lactate (lactic acid) can be formed, which is found in striated muscles, blood (more precisely, in red blood cells) and the human brain. An important element in the functioning of the heart and nervous system. A decarboxylation reaction occurs, that is, the cleavage of the carboxyl (acidic) group of amino acids, during which coenzyme A is formed - it performs the function of transporting carbon in various metabolic processes. When combined with a molecule of oxaloacetate (oxalic acid), citrate is obtained, which appears in buffer exchanges, i.e., “itself” carries useful substances in our body and helps them to be absorbed. At this stage, coenzyme A is completely released, plus we get a water molecule. This reaction is irreversible.
- The second stage is characterized by dehydrogenation (cleavage of water molecules) from the citrate, giving us cis-aconitate (aconitic acid), which helps in the formation of isocitrate. By the concentration of this substance, for example, you can determine the quality of fruit or fruit juice.
- Third stage. Here the carboxyl group is separated from isocitric acid, resulting in ketoglutaric acid. Alpha-ketoglutarate is involved in improving the absorption of amino acids from incoming food, improves metabolism and prevents stress. NADH is also formed - a substance necessary for the normal course of oxidative and metabolic processes in cells.
- At the next stage, when the carboxyl group is separated, succinyl-CoA is formed, which is an essential element in the formation of anabolic substances (proteins, etc.). The process of hydrolysis occurs (combination with a water molecule) and ATP energy is released.
- At subsequent stages the cycle will begin to close, i.e. The succinate will again lose a water molecule, which turns it into fumarate (a substance that promotes the transfer of hydrogen to coenzymes). Water joins the fumarate to form malate (malic acid), which oxidizes, which again leads to the appearance of oxaloacetate. Oxaloacetate, in turn, acts as a catalyst in the above processes; its concentrations in cell mitochondria are constant, but rather low.
Thus, we can highlight the most important functions of this cycle:
- energy;
- anabolic (synthesis of organic substances - amino acids, fatty proteins, etc.);
- catabolic: the transformation of certain substances into catalysts - elements that contribute to energy production;
- transport, mainly transporting hydrogen involved in cell respiration.
Brief historical information
Our favorite cycle is the TCA cycle, or the tricarboxylic acid cycle - life on Earth and under the Earth and in the Earth... Stop, in general this is the most amazing mechanism - it is universal, it is a way of oxidizing the breakdown products of carbohydrates, fats, proteins in the cells of living organisms, as a result We get energy for the activities of our body.
This process was discovered by Hans Krebs himself, for which he received the Nobel Prize!
He was born in August 25 - 1900 in the German city of Hildesheim. He received a medical education from the University of Hamburg and continued biochemical research under the leadership of Otto Warburg in Berlin.
In 1930, together with his student, he discovered the process of neutralizing ammonia in the body, which was present in many representatives of the living world, including humans. This cycle is the urea cycle, which is also known as the Krebs cycle #1.
When Hitler came to power, Hans emigrated to Great Britain, where he continues to study science at the Universities of Cambridge and Sheffield. Developing the research of the Hungarian biochemist Albert Szent-Györgyi, he received an insight and made the most famous Krebs cycle No. 2, or in other words, the “Szent-Györgyö – Krebs cycle” - 1937.
The research results are sent to the journal Nature, which refuses to publish the article. Then the text flies to the magazine "Enzymologia" in Holland. Krebs received the Nobel Prize in 1953 in physiology or medicine.
The discovery was surprising: in 1935 Szent-Györgyi found that succinic, oxaloacetic, fumaric and malic acids (all 4 acids are natural chemical components of animal cells) enhance the oxidation process in the pectoral muscle of the pigeon. Which was shredded.
It is in it that metabolic processes occur at the highest speed.
F. Knoop and K. Martius in 1937 found that citric acid is converted into isocitric acid through an intermediate product, cis - aconitic acid. In addition, isocitric acid could be converted into a-ketoglutaric acid, and that into succinic acid.
Krebs noticed the effect of acids on the absorption of O2 by the pectoral muscle of a pigeon and identified an activating effect on the oxidation of PVC and the formation of Acetyl-Coenzyme A. In addition, the processes in the muscle were inhibited by malonic acid, which is similar to succinic acid and could competitively inhibit enzymes whose substrate is succinic acid .
When Krebs added malonic acid to the reaction medium, the accumulation of a-ketoglutaric, citric and succinic acids began. Thus, it is clear that the combined action of a-ketoglutaric and citric acids leads to the formation of succinic acid.
Hans examined more than 20 other substances, but they did not affect oxidation. Comparing the data obtained, Krebs obtained a cycle. At the very beginning, the researcher could not say for sure whether the process began with citric or isocitric acid, so he called it the “tricarboxylic acid cycle.”
Now we know that the first is citric acid, so the correct name is the citrate cycle or the citric acid cycle.
In eukaryotes, TCA cycle reactions occur in mitochondria, while all enzymes for catalysis, except 1, are contained in a free state in the mitochondrial matrix; the exception is succinate dehydrogenase, which is localized on the inner membrane of the mitochondrion and is embedded in the lipid bilayer. In prokaryotes, the reactions of the cycle occur in the cytoplasm.
Let's meet the participants of the cycle:
1) Acetyl Coenzyme A:- acetyl group
- coenzyme A - Coenzyme A:
2) PIKE – Oxaloacetate - Oxaloacetic acid:
seems to consist of two parts: oxalic and acetic acid.
3-4) Citric and Isocitric acids:
5) a-Ketoglutaric acid:
6) Succinyl-Coenzyme A:
7) Succinic acid:
8) Fumaric acid:
9) Malic acid:
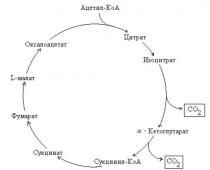
How do reactions occur? In general, we are all accustomed to the appearance of the ring, which is shown below in the picture. Below everything is described step by step:
1. Condensation of Acetyl Coenzyme A and Oxaloacetic acid ➙ citric acid.
The transformation of Acetyl Coenzyme A begins with condensation with Oxaloacetic acid, resulting in the formation of citric acid.
The reaction does not require the consumption of ATP, since the energy for this process is provided as a result of hydrolysis of the thioether bond with Acetyl Coenzyme A, which is high-energy:
2. Citric acid passes through cis-aconitic acid into isocitric acid.
Isomerization of citric acid into isocitric acid occurs. The conversion enzyme - aconitase - first dehydrates citric acid to form cis-aconitic acid, then connects water to the double bond of the metabolite, forming isocitric acid:
3. Isocitric acid is dehydrogenated to form α-ketoglutaric acid and CO2.
Isocitric acid is oxidized by a specific dehydrogenase, the coenzyme of which is NAD.
Simultaneously with oxidation, decarboxylation of isocitric acid occurs. As a result of transformations, α-ketoglutaric acid is formed.
4. Alpha-ketoglutaric acid is dehydrogenated by ➙ succinyl-coenzyme A and CO2.
The next stage is the oxidative decarboxylation of α-ketoglutaric acid.
Catalyzed by the α-ketoglutarate dehydrogenase complex, which is similar in mechanism, structure and action to the pyruvate dehydrogenase complex. As a result, succinyl-CoA is formed.
5. Succinyl coenzyme A ➙ succinic acid.
Succinyl-CoA is hydrolyzed to free succinic acid, the energy released is stored by the formation of guanosine triphosphate. This stage is the only one in the cycle at which energy is directly released.
6. Succinic acid is dehydrogenated ➙ fumaric acid.
The dehydrogenation of succinic acid is accelerated by succinate dehydrogenase, its coenzyme is FAD.
7. Fumaric acid is hydrated ➙ malic acid.
Fumaric acid, which is formed by dehydrogenation of succinic acid, is hydrated and malic acid is formed.
8. Malic acid is dehydrogenated ➙ Oxalic-acetic acid - the cycle closes.
The final process is dehydrogenation of malic acid, catalyzed by malate dehydrogenase;
The result of the stage is the metabolite with which the tricarboxylic acid cycle begins - Oxalic-Acetic acid.
In reaction 1 of the next cycle, another quantity of Acetyl Coenzyme A will enter.
How to remember this cycle? Just!
1) A very figurative expression:A Whole Pineapple and a Piece of Soufflé Is Actually My Lunch Today, which corresponds to - citrate, cis-aconitate, isocitrate, (alpha-)ketoglutarate, succinyl-CoA, succinate, fumarate, malate, oxaloacetate.
2) Another long poem:
PIKE ate acetate, it turns out citrate,
Through cisaconitate it will become isocitrate.
Having given up hydrogen to NAD, it loses CO2,
Alpha-ketoglutarate is extremely happy about this.
Oxidation is coming - NAD has stolen hydrogen,
TDP, coenzyme A takes CO2.
And the energy barely appeared in succinyl,
Immediately ATP was born and what remained was succinate.
Now he got to FAD - he needs hydrogen,
The fumarate drank from the water and turned into malate.
Then NAD came to malate, acquired hydrogen,
The PIKE showed up again and quietly hid.
3) The original poem - in short:
PIKE ACETYL LIMONIL,
But the horse was afraid of narcissus,
He is above him ISOLIMON
ALPHA - KETOGLUTARASED.
SUCCINALIZED WITH COENZYME,
AMBER FUMAROVO,
Stored up some APPLES for the winter,
Turned into a PIKE again.
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0