Carboxyl group (carboxyl) -COOH is a functional monovalent group that is part of carboxylic acids and determines their acidic properties.
The structure of the carboxyl group
The carboxyl group combines two functional groups - carbonyl (>C=O) and hydroxyl (-OH), mutually influencing each other.
The acidic properties of carboxylic acids are due to a shift in electron density to carbonyl oxygen and the resulting additional (compared to alcohols) polarization of the O-H bond.
In an aqueous solution, carboxylic acids dissociate into ions:
R-COOH = R-COO − + H +
Solubility in water and high boiling points of acids are due to the formation of intermolecular hydrogen bonds.
With increasing molecular weight, the solubility of acids in water decreases.
| Benzene | This is a draft article on organic chemistry. You can help the project by adding to it. |
Write a review about the article "Carboxyl group"
An excerpt characterizing the carboxyl group
– Svetodar, Sever... What happened to him? How did the son of Radomir and Magdalena live his life on Earth?..The North began to think... Finally, taking a deep breath, as if throwing off the obsession of the past, he began his next exciting story...
– After the crucifixion and death of Radomir, Svetodar was taken to Spain by the Knights of the Temple to save him from the bloody clutches of the “holy” church, which, no matter the cost, tried to find and destroy him, since the boy was the most dangerous living witness, and also , the direct successor of Radomir’s Tree of Life, which was supposed to someday change our world.
Svetodar lived and learned about his surroundings in the family of a Spanish nobleman, who was a faithful follower of the teachings of Radomir and Magdalene. To their great sadness, they did not have their own children, so the “new family” received the boy very cordially, trying to create for him as comfortable and warm a home environment as possible. They called him Amori (which meant dear, beloved), since it was dangerous for Svyatodar to be called by his real name. It sounded too unusual for someone else’s ears, and it was more than unreasonable to risk Svetodar’s life because of this. So Svetodar became Amory’s boy for everyone else, and only his friends and his family called him by his real name. And then, only when there were no strangers nearby...
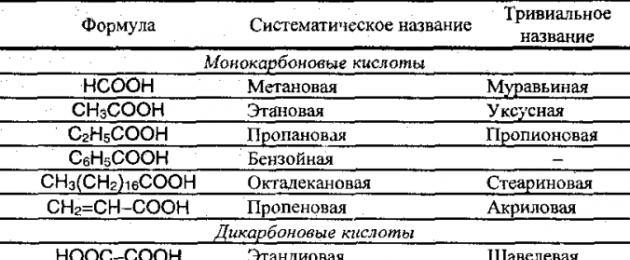
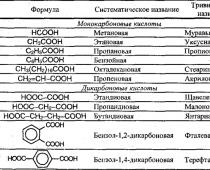
Carboxylic acids are compounds that contain a carboxyl group:
Carboxylic acids are distinguished:
- monobasic carboxylic acids;
- dibasic (dicarboxylic) acids (2 groups UNS).
Depending on their structure, carboxylic acids are distinguished:
- aliphatic;
- alicyclic;
- aromatic.
Examples of carboxylic acids.


Preparation of carboxylic acids.
1. Oxidation of primary alcohols with potassium permanganate and potassium dichromate:

2. Hybrolysis of halogen-substituted hydrocarbons containing 3 halogen atoms per carbon atom:
3. Preparation of carboxylic acids from cyanides:
When heated, the nitrile hydrolyzes to form ammonium acetate:
When acidified, acid precipitates:
4. Use of Grignard reagents:
5. Hydrolysis of esters:
6. Hydrolysis of acid anhydrides:
7. Specific methods for obtaining carboxylic acids:
Formic acid is produced by heating carbon(II) monoxide with powdered sodium hydroxide under pressure:
Acetic acid is produced by the catalytic oxidation of butane with atmospheric oxygen:
Benzoic acid is obtained by oxidation of monosubstituted homologues with a solution of potassium permanganate:
Canniciaro's reaction. Benzaldehyde is treated with 40-60% sodium hydroxide solution at room temperature.

Chemical properties of carboxylic acids.
In an aqueous solution, carboxylic acids dissociate:

The equilibrium is shifted strongly to the left, because carboxylic acids are weak.
Substituents affect acidity due to an inductive effect. Such substituents pull electron density towards themselves and a negative inductive effect (-I) occurs on them. The withdrawal of electron density leads to an increase in the acidity of the acid. Electron-donating substituents create a positive inductive charge.

1. Formation of salts. Reaction with basic oxides, salts of weak acids and active metals:

Carboxylic acids are weak, because mineral acids displace them from the corresponding salts:
2. Formation of functional derivatives of carboxylic acids:
3. Esters when heating an acid with an alcohol in the presence of sulfuric acid - esterification reaction:
4. Formation of amides, nitriles:
3. The properties of acids are determined by the presence of a hydrocarbon radical. If the reaction occurs in the presence of red phosphorus, it forms the following product:
4. Addition reaction.

8. Decarboxylation. The reaction is carried out by fusing an alkali with an alkali metal salt of a carboxylic acid:
9. Dibasic acid is easily eliminated CO 2 when heated:
Additional materials on the topic: Carboxylic acids.
Chemistry calculators |
|
| Chemistry online on our website to solve problems and equations. | |
The elements included in the carboxyl group have different electronegativity values: C(sp 2) – 2.69; O – 3.5 and H – 2.2. The electron density is shifted towards oxygen, which leads to polarization of bonds. The carboxyl group is a planar conjugated system in which p, p-conjugation occurs when the p-orbital of the oxygen atom of the hydroxyl group interacts with the p-bond. The presence of p, p-conjugation in the carboxyl group of carboxylic acids contributes to the uniform distribution of the negative charge in the carboxylate ion formed when a proton is removed.
The presence of p,p-conjugation in the carboxyl group of carboxylic acids significantly increases the acidic properties of carboxylic acids compared to alcohols. The oxygen of the OH group experiences some electron deficiency, so it causes a shift in electron density from the hydrogen atom to itself. The hydrogen atom becomes mobile and can leave in the form of a proton. Proton mobility promotes the formation of carboxylic acid salts (S E reaction mechanism).
For carboxylic acids, the most typical reactions are nucleophilic substitution of the OH group in the carboxyl (S N mechanism). In reactions in which an atom (or group of atoms) is replaced by a nucleophile, nucleophilic substitution S N occurs. Nucleophiles are electron donors: OH -, RO -, CN -, -NH 2, R-O-R, R-OH, etc. In S N reactions, an atom (or groups of atoms) with a negative charge is removed from the molecule and replaced by a nucleophile:

The more stable the leaving ion (:X), the easier the nucleophilic substitution reactions occur. S N reactions with carboxylic acids occur in the presence of an acid catalyst. Reactions of nucleophilic substitution of hydroxyl at the sp 2 -hybridized carbon atom lead to the formation of functional derivatives of carboxylic acids:
1. Esterification reaction. Substitution of the hydroxyl in the carboxyl with an alcohol residue leads to the formation of esters. The esterification reaction occurs when acids are heated with alcohols in the presence of water-removing substances:

CH 3 COOH + C 2 H 5 OH ↔ CH 3 CO-OS 2 H 5 + H 2 O
ethyl acetate
The reaction is reversible and proceeds until equilibrium is established. The shift of equilibrium to the right occurs due to the removal of one of the final products from the reaction mixture.
Hydrolysis of esters (as opposed to the esterification reaction) can be carried out in both acidic and alkaline environments.
2. Acid halides- these are derivatives in which the hydroxyl group of the carboxyl is replaced by a halogen. Acid halides are formed by the action of phosphorus halides (PCl 5, PCl 3) or thionyl chloride (SOCl 2) on an acid:
Acid halides are highly active nucleophiles and are used in various syntheses as active acylating agents to introduce an acid residue into the molecule of a substance.
3.Formation of acid anhydrides– replacement of hydroxyl in carboxyl with an acid residue.
Anhydrides of monocarboxylic acids are most often prepared by the reaction of acid chlorides and sodium salts of these acids.
4. Formation of acid amides- replacement of hydroxyl in carboxyl with an ammonia residue - amino group - NH 2

The reaction proceeds better if you act on acid halides with ammonia.
5. Formation reactions are quite common in the human body. thioethers. Thus, tissues contain the thiol HS–KoA (coenzyme A - acylation coenzyme), which can react with carboxylic acids according to the equation:
CH 3 COOH + HS-KoA → H 2 O + CH 3 CO-S-CoA
Reactions of this kind are catalyzed by complex enzyme systems and result in the formation of thioesters, in this form carboxylic acids are used in metabolic reactions.
Decarboxylation is one of the most important reactions of carboxylic acids. Unsubstituted monocarboxylic acids are difficult to decarboxylate.
Dibasic carboxylic acids are easily decarboxylated when heated:
HOOC-COOH → HCOOH + CO 2
HOOC–CH 2 –COOH → CH 3 COOH + CO 2
Enzymatic decarboxylation of α- and β-oxoacids, as well as α-amino acids in the body is of great importance.
In aqueous solutions, carboxylic acids dissociate:
CH 3 COOH ↔ CH 3 COO - + H +
All carboxylic acids are weak electrolytes (HCOOH - medium strength). Carboxylic acids form salts in electrophilic substitution reactions:
CH 3 COOH + NaOH → CH 3 COONa + H 2 O
Salts of oxalic acid (oxalates) are poorly soluble and often form kidney and bladder stones (oxalate stones). These salts include calcium oxalate.
4. Illustrative material: presentation
5. Literature:
Main literature:
2. Seitembetov T.S. Chemistry: textbook - Almaty: EVERO LLP, 2010. - 284 p.
3. Bolysbekova S. M. Chemistry of biogenic elements: textbook - Semey, 2012. - 219 p. : silt
4. Verentsova L.G. Inorganic, physical and colloidal chemistry: textbook - Almaty: Evero, 2009. - 214 p. : ill.
5. Physical and colloidal chemistry /Under the editorship of A.P. Belyaev. - M.: GEOTAR MEDIA, 2008
6. Verentseva L.G. Inorganic, physical and colloidal chemistry, (verification tests) 2009
Additional literature:
Test "Pyaterochka"
(Some questions have more than one possible answer.)
1. Which of the following acids are organic?
A) Ant; b) nitrogen;
B) sulfur; d) lemon.
2. Why are ant bites painful?
A) Burn with formic acid;
B) secrete poison;
C) corroded by formic alkali;
D) sharp teeth pierce.
3. What are salts of carboxylic acids called?
A) acetates; b) bustylates;
B) propylates; d) postulates.
4. What name for an acid does not exist?
A) Lemon; b) oxaline;
B) wine; d) grape.
5. What acids are vitamins?
A) Nicotine; b) ascorbic acid;
B) acetylsalicylic acid; d) amber.
Lecture No. 5
1. Topic: Carbohydrates.
2. Purpose: To develop knowledge about the classification, structure, and chemical properties of carbohydrates. Studying the topic promotes and forms knowledge about the structure and properties of carbohydrates, as the basis for understanding their metabolism in a living organism
3. Lecture abstracts:
3.1. Lecture outline
1. Carbohydrates, classification, carbonyl and projection formulas of Fischer, Colley-Tollens formulas, Haworth formulas.
2. Monosaccharides - structure, isomerism, tautomerism.
3. Chemical properties of monosaccharides
4. Disaccharides: maltose, cellobiose, lactose, sucrose.
5. Polysaccharides: homopolysaccharides and heteropolysaccharides.
6. Biological significance of carbohydrates.
3.2. Lecture abstracts
Carbohydrates are organic compounds widespread in living nature. The term “carbohydrates” was proposed in 1844 by the chemist K. Schmidt (Russia) due to the fact that the majority of representatives of this class in composition corresponded to the combination of carbon with water, for example: C 6 H 12 O 6 → 6 C + 6 H 2 O; Сn(H2O)n.
The carbohydrate class is divided into two groups:
- simple carbohydrates (monosaccharides) or monosaccharides
- complex carbohydrates (homopolysugars and heteropolysugars)
Simple carbohydrates cannot be hydrolyzed, but complex carbohydrates are hydrolyzed to form simple carbohydrates.
Monosaccharides- these are heterofunctional compounds. They are aldehydes and ketones of polyhydric alcohols (or their cyclic hemiacetals). Depending on the presence of an aldehyde or ketone group, aldoses and ketoses are distinguished. Monosaccharides contain from 3 to 10 carbon atoms per molecule.
Isomerism of monosaccharides is associated with the presence of: 1) aldehyde and ketone groups; 2) asymmetric carbon atoms (optical isomerism). The belonging of monosaccharides to the D- or L-series is determined by the M.A. rule. Rozanova: according to the configuration of the asymmetric carbon atom farthest from the senior (carbonyl) group (pentose C-4, hexose C-5).
Carbonyl formulas of hexoses:
D-glucose D-mannose D-galactose D-fructose
Monosaccharides exist in the form of acyclic (carbonyl forms) and cyclic hemiacetal forms. Acyclic and cyclic forms of monosaccharides are isomers with respect to each other. This type of isomerism is called cyclo-oxo-tautomerism.
Cyclic (Haworth) formulas of hexoses:




a-D-glucose b-D-glucose a-D-galactose b-D-fructose
For sugars in cyclic form it is possible conformational isomerism, associated with the spatial arrangement of the carbon atoms of the six-membered cycle. Reactions of hemiacetal hydroxyl. Monosaccharides having the structure of cyclic hemiacetals, when reacting with alcohols in the presence of an acid catalyst under anhydrous conditions, form complete acetals.

a-D-galactopyranose Methyl-a-D-galactopyranoside
Hemiacetal or glycosidic hydroxyl does not exhibit the properties of alcohols, but behaves specifically, forming O- and N-glycosides. Glycosides, like all acetals, hydrolyze in an acidic environment and are resistant to the action of dilute alkalis. Hydrolysis of glycosides is the reverse reaction of the formation of glycosides
Like polyhydric alcohols, monosaccharides dissolve copper(II) hydroxide, forming a blue chelate compound. This reaction is used to detect monosaccharides and glycosides.
Ethers are formed upon interaction with haloalkanes. Simultaneously with the alcohol hydroxyl groups, the hemiacetal hydroxyl also reacts, resulting in the formation of a glycoside ether.



Ether Esters: α-glucose and α-fructose phosphates
Esters are formed by the esterification reaction of OH groups under the action of oxygen-containing acids (organic and inorganic) or their anhydrides. Phosphoric acid esters - phosphates - are of biological importance.
Recovery the carbonyl group of monosaccharides with hydrogen on nickel or palladium leads to the formation of polyhydric alcohols: glucose - sorbitol (D-glucite), mannose - mannitol, D-xylose - xylitol, galactose - dulcite. Xylitol and sorbitol are sweet-tasting crystalline substances that are highly soluble in water and are used as sugar substitutes for diabetes. Sorbitol is an intermediate product in the industrial production of ascorbic acid (vitamin C).
Oxidation of monosaccharides.
The scheme of the chemical reaction C x (H 2 O) y + xO 2 ® xCO 2 + yH 2 O + Q corresponds to the process of complete oxidation (combustion) of carbohydrates.
Depending on the conditions of oxidation of monosaccharides, glycan, glycaric and glycuronic acids are formed.
Neutral environment. Only the aldehyde group is oxidized into a carboxyl group in a neutral or acidic environment (mild oxidation). Oxidation of glucose with bromine water in the presence of chalk (used to neutralize the resulting HBr) at room temperature leads to the formation of gluconic (glyconic) acid:


gluconic acid glucaric acid glucuronic acid
Gluconic acid in the form of calcium salts (calcium glucanate) is widely used in practical medicine (for allergies, increased permeability of blood vessels, etc.).
Acidic environment. When monosaccharides are heated with dilute nitric acid, simultaneously with the aldehyde group, the primary alcohol group at the other end of the monosaccharide chain is oxidized, which leads to the formation of dibasic hydroxy acids (glycaric acids). Glucose is glucaric acid.
Alkaline environment. When oxidized in an alkaline environment with heating, monosaccharides undergo profound changes with the splitting of the carbon chain. In this case, such oxidizing agents as silver oxide Ag 2 O (silver mirror reaction) and copper (II) hydroxide Cu(OH) 2 are reduced to metallic silver and copper (I) oxide (Trommer test).
In the tissues of the human body, during the oxidation of glucose, glucuronic acid can be formed (the aldehyde group is protected and preserved, only the primary alcohol group is oxidized). The oxidation of galactose produces galacturonic acid. Uronic acids play a protective role in the human liver, participating in the neutralization of toxic substances coming from the outside or formed as a result of vital activity. Decarboxylation of uronic acids leads to the formation of pentoses (glucuronic acid → xylose + CO 2).
Monosaccharide derivatives
Deoxysugars are derivatives of monosaccharides in which one or two HO groups are replaced by a hydrogen atom, for example 2-deoxyribose.
Amino sugar– are formed on the basis of monosaccharides, in the molecules of which the OH group of the second link is replaced by an amino group - NH 2, for example β,D-glucosamine.
Neuramic acid . Obtained as a result of aldol condensation of PVK (1) and D-mannosamine (2):

Sialic acids. They are N-acetyl derivatives of neuraminic acid. Acylation occurs with an acetyl or hydroxyacetyl residue.
Complex carbohydrates- these are compounds whose hydrolysis produces monosaccharides and their derivatives. Depending on the structure and number of structural components, complex carbohydrates are usually divided into oligosaccharides(2-10 monoz) and polysaccharides.
Disaccharides formed from two monosaccharides. Their general empirical formula is C 12 H 22 O 11. The most important disaccharides are maltose, lactose, sucrose, cellobiose. Maltose(malt sugar) - a reducing disaccharide - consists of residues of 2 molecules of D-glucopyranose linked by an alpha-1,4-glycosidic bond; upon hydrolysis, it is split into two D-glucoses (D-glucopyranoses).
Lactose(milk sugar) is a reducing disaccharide. Contained in the milk of mammals. Upon hydrolysis, it forms 2 monosaccharides: β-D-galactopyranose (β-D-galactose) and D-glucose.
Sucrose(cane sugar, beet sugar) is the most famous and widespread sugar. During hydrolysis, sucrose is broken down into α-glucose and β-fructose. The α-1, β-2-glycosidic bond is formed by the hemiacetal hydroxyls of α-glucose and β-fructose, so there is no possibility for cyclo-oxotautomerism. Sucrose is a non-reducing disaccharide.
Cellobiose– a reducing disaccharide, consists of residues of 2 molecules of β-D-glucopyranose linked by a beta-1,4-glycosidic bond. It is obtained by hydrolysis of cellulose (fiber).
Polysaccharides- These are high-molecular carbohydrates, chemically related to polyglycosides, i.e. are products of polycondensation of monosaccharides linked by glycosidic bonds.
Polysaccharide chains can be branched (amylopectin, glycogen) and unbranched, that is, linear (fiber, amylose).
Based on their composition, polysaccharides are divided into
1. homopolysaccharides- biopolymers formed from residues of one monosaccharide;
2. heteropolysaccharides- biopolymers formed from residues of various monosaccharides.
They all have a common name: glycans.
Starch– is a reserve polysaccharide in most plants, in which it is formed due to the reaction of photosynthesis. Starch is not homogeneous in structure - it is a mixture of two polysaccharides: amylose and amylopectin in a ratio of 10-20% to 80-90%. Amylose consists of a-D-glucopyranose residues linked by a(1®4)-glycosidic bonds. The amylose macromolecule may contain 200 to 1000 monomer residues. Amylopectin is a homopolysaccharide with a branched structure, in which the linear chain of a-D-glucopyranose residues is built through a(1®4) glycosidic bonds, and the branching elements are formed through a(1®6) glycosidic bonds. Between the branch points there are 20 to 25 glucose residues; molecular weight of amylopectin is 1-6 million units.
4. Illustrative material: presentation
5. Literature:
Main literature:
1. Bioorganic chemistry: textbook. Tyukavkina N.A., Baukov Yu.I. 2014
- Seitembetov T.S. Chemistry: textbook - Almaty: EVERO LLP, 2010. - 284 p.
- Bolysbekova S. M. Chemistry of biogenic elements: textbook - Semey, 2012. - 219 p. : silt
- Verentsova L.G. Inorganic, physical and colloidal chemistry: textbook - Almaty: Evero, 2009. - 214 p. : ill.
- Physical and colloidal chemistry / Edited by A.P. Belyaev. - M.: GEOTAR MEDIA, 2008
- Verentseva L.G. Inorganic, physical and colloidal chemistry, (verification tests) 2009
Additional literature:
- Ravich-Scherbo M.I., Novikov V.V. Physical and colloidal chemistry. M. 2003.
2. Slesarev V.I. Chemistry. Fundamentals of living chemistry. St. Petersburg: Khimizdat, 2001
3. Ershov Yu.A. General chemistry. Biophysical chemistry. Chemistry of biogenic elements. M.: VSh, 2003.
4. Asanbaeva R.D., Ilyasova M.I. Theoretical foundations of the structure and reactivity of biologically important organic compounds. Almaty, 2003.
- Guide to laboratory classes in bioorganic chemistry, ed. ON THE. Tyukavkina. M., Bustard, 2003.
- Glinka N.L. General chemistry. M., 2003.
- Ponomarev V.D. Analytical chemistry part 1, 2 2003
6. Test questions (feedback):
- What percentage of the daily intake in a balanced diet should be carbohydrates?
- What does a lack of carbohydrates in food lead to?
- What does excess carbohydrates in food lead to?
Lecture No. 6
1. Topic:α-amino acids. Peptides. Squirrels.
2. Purpose: study the structure, properties, pathways of formation, α-amino acids in the body. Studying the topic contributes to, forms knowledge of the composition, structure and biological role of α-amino acids, peptides and proteins, to study the biological functions of proteins at the molecular level
3. Lecture abstracts:
3.1. Lecture outline
1. Structure of amino acids, classification. Isomerism
2. Pathways for the formation of amino acids in a living organism.
3. Physical and chemical properties of amino acids.
4. The concept of peptides, their biological significance
5. Structure of the peptide bond
6. Proteins, classification, levels of organization, biological role
7. Hemoglobin is a representative of chromoproteins
8. Qualitative reactions to α-amino acids and proteins
Lecture abstracts
Amino acids (AMA) are derivatives of carboxylic acids, in the radical of which one or more hydrogen atoms are replaced by amino groups.
General formula of AMK:
Or alpha amino acid
Amino acid molecules can contain several NH 2 and -COOH.
Based on the position of the amino group in the carbon chain relative to the carboxyl group, α-, β-, γ-amino acids are distinguished. α-amino acids (α-ama) are protein monomers.
Classification of alpha amino acids.
1. Based on the number of amino and carboxyl groups, they distinguish: monoaminomonocarboxylic acids (glycine, alanine, valine, leucine, isoleucine, serine, threonine, cysteine, methionine, phenylalanine, tyrosine, histidine, tryptophan); monoaminodicarboxylic acids (glutamic acid, aspartic acid, cystine); diaminomonocarboxylic acids (lysine, arginine).
2. By the presence of functional groups in the radical: hydroxyamino acids (serine, threonine); sulfur-containing amino acids (methionine, cysteine, cystine).
3. By the nature of the radical: aliphatic amino acids (glycine, alanine, leucine, etc.); aromatic amino acids (phenylalanine, tyrosine); heterocyclic aromatic amino acids (tryptophan, histidine); heterocyclic imino acids (proline, hydroxyproline).
Amino acid isomerism is associated with the position of functional groups and the structure of the carbon skeleton. All alpha amino acids that make up proteins (except GLI) are optically active substances, because contain an asymmetric carbon atom and exist in the form of enantiomers - D– and L- optical isomers. Animal proteins contain L-ABA.
The carboxyl group combines two functional groups - carbonyl and hydroxyl, which mutually influence each other. This influence is transmitted through the interface system sp 2 atoms O–C–O.
The electronic structure of the –COOH group gives carboxylic acids characteristic chemical and physical properties.
1. The shift of electron density to the carbonyl oxygen atom causes additional (compared to alcohols and phenols) polarization of the O–H bond, which determines the mobility of the hydrogen atom ( acid properties).
In an aqueous solution, carboxylic acids dissociate into ions:
However, carboxylic acids in general are weak acids: in aqueous solutions their salts are highly hydrolyzed.
Video experiment "Carboxylic acids are weak electrolytes."
2. Reduced electron density (δ+) on the carbon atom in the carboxyl group makes reactions possible nucleophilic substitution groups -OH.
3. The -COOH group, due to the positive charge on the carbon atom, reduces the electron density on the hydrocarbon radical associated with it, i.e. is in relation to him electron-withdrawing deputy In the case of saturated acids, the carboxyl group exhibits -I -Effect, and in unsaturated (for example, CH 2 =CH-COOH) and aromatic (C 6 H 5 -COOH) - -I And -M -effects.
4. The carboxyl group, being an electron acceptor, causes additional polarization of the C–H bond in the neighboring (α-) position and increases the mobility of the α-hydrogen atom in substitution reactions at the hydrocarbon radical.
See also "Reaction centers in carboxylic acid molecules".
The hydrogen and oxygen atoms in the carboxyl group -COOH are capable of forming intermolecular hydrogen bonds, which largely determines physical properties carboxylic acids.
Due to the association of molecules, carboxylic acids have high boiling and melting points. Under normal conditions they exist in a liquid or solid state.
For example, the simplest representative is formic acid HCOOH - a colorless liquid with bp. 101 °C, and pure anhydrous acetic acid CH 3 COOH, when cooled to 16.8 °C, turns into transparent crystals resembling ice (hence its name glacial acid).
Video experiment "Glacial acetic acid".
The simplest aromatic acid - benzoic acid C 6 H 5 COOH (mp 122.4 ° C) - easily sublimes, i.e. turns into a gaseous state, bypassing the liquid state. When cooled, its vapors sublimate into crystals. This property is used to purify a substance from impurities.
Video experiment "Sublimation of benzoic acid."
The solubility of carboxylic acids in water is due to the formation of intermolecular hydrogen bonds with the solvent:
Lower homologs C 1 -C 3 are miscible with water in any ratio. As the hydrocarbon radical increases, the solubility of acids in water decreases. Higher acids, for example, palmitic C 15 H 31 COOH and stearic C 17 H 35 COOH, are colorless solids that are insoluble in water.
The carboxyl group combines two functional groups - carbonyl and hydroxyl, which mutually influence each other:
The acidic properties of carboxylic acids are due to a shift in electron density to carbonyl oxygen and the resulting additional (compared to alcohols) polarization of the O–H bond.
In an aqueous solution, carboxylic acids dissociate into ions:
Carboxylic acid derivatives: salts, esters, acid chlorides, anhydrides, amides, nitriles, their preparation.
Carboxylic acids exhibit high reactivity. They react with various substances and form a variety of compounds, among which functional derivatives are of great importance, i.e. compounds obtained as a result of reactions at the carboxyl group.
1. Formation of salts
a) when interacting with metals:
2RCOOH + Mg ® (RCOO) 2 Mg + H 2
b) in reactions with metal hydroxides:
2RCOOH + NaOH ® RCOONa + H 2 O
2. Formation of esters R"–COOR":

The reaction of forming an ester from an acid and an alcohol is called the esterification reaction (from lat. ether- ether).
3. Formation of amides:

Instead of carboxylic acids, their acid halides are more often used:

Amides are also formed by the interaction of carboxylic acids (their acid halides or anhydrides) with organic ammonia derivatives (amines):

Amides play an important role in nature. Molecules of natural peptides and proteins are built from a-amino acids with the participation of amide groups - peptide bonds
Nitriles are organic compounds of the general formula R-C≡N, considered as derivatives of carboxylic acids (dehydration products of amides) and referred to as derivatives of the corresponding carboxylic acids, for example, CH 3 C≡N - acetonitrile (nitrile of acetic acid), C 6 H 5 CN - benzonitrile (benzoic acid nitrile).
Carboxylic acid anhydrides can be considered as the condensation product of two -COOH groups:
R 1 -COOH + HOOC-R 2 = R 1 -(CO)O(OC)-R 2 + H 2 O
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0