Instructions
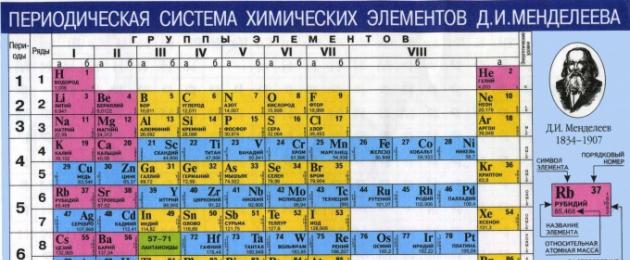
If you carefully examine the table of Dmitry Ivanovich Mendeleev, you can see that it has the appearance of an apartment high-rise building in which there are “tenants” - elements. Each of them has a surname () and a chemical name. Moreover, each of the elements lives in its own apartment, and therefore has. This information is presented in all cells of the table.
However, there is another figure there, which at first glance is completely incomprehensible. Moreover, it is indicated with several values after the decimal point, which is done for greater accuracy. It is this number that you need to pay attention to, because this is the relative atomic mass. Moreover, this is a constant value that does not need to be memorized and can be found in the table. By the way, even on the Unified State Examination according to D.I. Mendeleev is reference material, available for use, and everyone has it in an individual package - KIM.
The molecular mass, or rather the relative mass of a substance, is denoted by the letters (Mr) and is made up of the relative atomic masses (Ar) of the elements that form the molecule. Relative atomic mass is exactly that mysterious number that appears in every cell of the table. For calculations, these values must be rounded to a whole number. The only exception is the chlorine atom, whose relative atomic mass is 35.5. This characteristic has no units of measurement.
Example 1. Find the molecular mass(KOH)
A potassium hydroxide molecule consists of one potassium atom (K), one oxygen atom (O), and one hydrogen atom (H). Therefore, we find:
Mr (KOH) = Ar (K) + Ar (O) + Ar (H)
Hence: Mr (KOH) = 39 + 16 + 1 = 56
Example 2. Find the molecular mass sulfuric acid (H2SO4 ash-two-es-o-four)
A sulfuric acid molecule consists of two hydrogen atoms (H), one sulfur atom (S) and four oxygen atoms (O). Therefore, we find:
Mr (H2SO4) = 2Ar (H) + Ar (S) + 4Ar (O)
According to the table D.I. Mendeleev we find the values of the relative atomic masses of elements:
Ar (K) = 39, Ar (O) = 16, Ar (H) = 1
Hence: Mr (H2SO4) = 2 x 2 + 32 + 4 x 16 = 98
Video on the topic
note
When performing calculations, multiplication or division is performed first, and only then addition or subtraction.
When determining relative atomic mass, round off the values found in the D.I. table. Mendeleev to an integer
Sources:
- how to calculate molecular weight
- Molecular mass determination
To find the molecular mass, find the molar mass substances in grams per mole, since these quantities are numerically equal. Or find mass particles of a molecule in atomic mass units, add their values and get the molecular mass. To find the molecular mass of a gas, you can use the Clapeyron-Mendeleev equation.
You will need
- For calculations you will need Mendeleev's periodic table, scales, thermometer, pressure gauge.
Instructions
Calculation using the periodic table. Determine the chemical formula of the substance under study. In the periodic table, find the chemical elements that make up the molecule. In the appropriate cells, find their atomic mass. If the table is a fraction, round it to the nearest whole number. If the same element occurs several times in a molecule, multiply it mass by the number of occurrences. Add up all the atoms. The result will be substances.
Calculation of molecular weight when converting from grams. If given the mass of one molecule in grams, multiply it by Avogadro's constant, which is 6.022 10^(23) 1/mol. The result will be substances in grams per mole. Its numerical value coincides with the molecular mass in atomic mass units.
Calculation of the molecular mass of an arbitrary gas. Take a cylinder of known volume measured in cubic meters, pump out the air from it and weigh it on a scale. Then pump gas into it, molecular mass which needs to be determined. Find again mass balloon. The difference between a gas cylinder and an empty cylinder will be equal to the mass of the gas, enter in grams. Measure the pressure with a pressure gauge (in) and the temperature with a thermometer, turning it to . To do this, add the number 273 to the degrees Celsius obtained as a result of the measurement. To find the molar mass gas, its mass multiply by temperature and the number 8.31 (universal gas constant). Sequentially divide the obtained result by the value of the gas pressure and its volume M=m 8.31 T/(P V). This measure, expressed in grams per mole, is the numerical molecular mass of a gas expressed in atomic mass units.
Video on the topic
Sources:
- molecular weight calculation
The relative molecular mass of a substance (or simply molecular mass) is the ratio of the mass of a given substance to 1/12 of the mass of one carbon atom (C). Find the relative molecular mass mass very easy.

You will need
- Periodic table and molecular weight table
Instructions
The relative mass of a substance is the sum of its atomic masses. In order to learn atomic mass one way or another, just look at the periodic table. It can be found on the cover of any book, or purchased separately at a bookstore. A pocket version or an A4 sheet is quite suitable for this. Any modern chemistry system is equipped with a full-scale wall-mounted periodic table.
Having learned atomic mass element, you can begin to calculate the molecular mass of the substance. This is easiest to show with an example:
It is required to calculate the molecular mass water (H2O). From the molecular formula it is clear that a water molecule consists of two H atoms and one O atom. Therefore, the calculation of the molecular mass of water can be reduced to the action:
1.008*2 + 16 = 18.016
Video on the topic
note
Atomic mass as a concept appeared in 1803, thanks to the works of the then famous chemist John Dalton. In those days, the mass of any atom was compared with the mass of a hydrogen atom. Further development This concept received in the works of another chemist - Berzelius, in 1818, when he proposed using an oxygen atom instead of a hydrogen atom. Since 1961, chemists in all countries have adopted the mass of 1/16 of an oxygen atom or the mass of 1/12 of a carbon atom as a unit of atomic mass. The latter is exactly indicated in the table chemical elements Mendeleev.
Helpful advice
When using the periodic table in the form in which it is presented in most chemistry textbooks and other reference books, one must understand that this table is a shortened version of the original periodic table. In its most complete version, a separate line is devoted to each chemical element.
The molecular mass of a substance means the total atomic mass of all chemical elements that are part of this substance. To calculate the molecular mass substances, no special effort is required.

You will need
- Mendeleev table.
Instructions
Now you need to take a closer look at any of the elements in this table. Under the name of any of the elements indicated in the table there is a numerical value. This is precisely the atomic mass of this element.
Now it is worth looking at several examples of molecular mass calculations, based on the fact that atomic masses are now known. For example, you can calculate the molecular weight of a substance such as water (H2O). A water molecule contains one oxygen atom (O) and two hydrogen atoms (H). Then, having found the atomic masses of hydrogen and oxygen using the periodic table, we can begin to calculate the molecular mass:2*1.0008 (after all, there are two hydrogens) + 15.999 = 18.0006 amu (atomic mass units).
Another . The next substance, molecular mass which can be calculated, let it be ordinary table salt (NaCl). As can be seen from the molecular formula, a molecule of table salt contains one Na atom and one Cl atom. In this case, it is calculated as follows: 22.99 + 35.453 = 58.443 a.m.u.
Video on the topic
note
I would like to note that the atomic masses of isotopes of various substances differ from the atomic masses in the periodic table. This is due to the fact that the number of neutrons in the nucleus of an atom and inside an isotope of the same substance is different, therefore the atomic masses are also noticeably different. Therefore, it is customary to denote isotopes of various elements by the letter of the given element, adding its mass number in the upper left corner. An example of an isotope is deuterium (“heavy hydrogen”), the atomic mass of which is not one, like an ordinary atom, but two.
Molar is weight one mole of a substance, that is, an amount that contains the same number of atoms as 12 grams of carbon. In another way, such a quantity is called Avogadro's number (or constant), in honor of the Italian scientist who first put forward the hypothesis. According to it, in equal volumes ideal gases(at the same temperatures and pressures) must be contained same number molecules.

We must firmly remember that one mole of any substance is approximately 6.022 * 1023 molecules (either atoms or ions) of this substance. Consequently, any quantity of any substance can be represented by elementary calculations in the form of a certain number of moles. Why was mola introduced at all? To make calculations easier. After all, the number of elementary (molecules, atoms, ions) even in the smallest sample of matter is simply colossal! Agree, it is much more convenient to express the amount of substances in moles rather than in huge ones with endless rows of zeros! Molar weight a substance is determined by adding the molar masses of all elements included in it, taking into account the indices. For example, you need to determine the molar mass of anhydrous sodium sulfate. First of all, write its chemical formula: Na2SO4. Do the calculations: 23*2 + 32 + 16*4 = 142 grams/mol. This will be the molar weight this salt. What if you need to determine the molar mass of a simple substance? The rule is absolutely the same. For example, molar weight oxygen O2 = 16*2 = 32 grams/mol, molar weight N2 = 14*2 = 28 grams/mol, etc. It is even easier to determine the molar mass of a molecule whose molecule consists of one atom. For example, molar weight sodium is 23/mol, silver is 108 grams/mol, etc. Of course, rounded values are used here to simplify calculations. If there is greater accuracy, it is necessary for the same sodium to consider its relative atomic mass to be not 23, but 22.98. We must also remember that the molar mass of a substance depends on its quantitative and qualitative composition. That's why different substances with the same number of moles they have different molar masses.
Video on the topic
Tip 6: How to Determine Relative Molecular Weight
The relative molecular mass of a substance is a value that shows how many times the mass of one molecule of a given substance is greater than 1/12 of the mass of the carbon isotope. In another way, it can be simply called molecular weight. How can you find the relative molecular mass?

You will need
- Mendeleev table.
Instructions
All you need for this is the Periodic Table and basic ability to do calculations. After all, relative molecular weight is the sum of the atomic masses of the elements that make up the composition you are interested in. Of course, taking into account the indices of each element. The atomic mass of each element is indicated in the Periodic Table along with other important information, and with very high accuracy. Rounded values are also quite suitable for these purposes.
Now take the Periodic Table and determine the atomic masses of each element included in its composition. There are three such elements: , sulfur, . Atomic mass (H) = 1, atomic mass of sulfur (S) = 32, atomic mass of oxygen (O) = 16. Taking into account the indices, sum up: 2 + 32 + 64 = 98. This is exactly the relative molecular mass of sulfuric acid. note that we're talking about about the approximate, rounded result. If for some reason accuracy is required, then you will have to take into account that the atomic mass of sulfur is not exactly 32, but 32.06, hydrogen is not exactly 1, but 1.008, etc.
note
If you don’t have the Periodic Table at hand, find out the relative molecular mass of a particular substance using chemistry reference books.
Helpful advice
The mass of a substance in grams, which is numerically equal to its relative molecular mass, is called a mole.
The relative molecular weight of a substance shows how many times a molecule of a given substance is heavier than 1/12 of a pure carbon atom. It can be found if its chemical formula is known using Mendeleev's periodic table of elements. Otherwise, use other methods to find molecular mass, keeping in mind that it is numerically equal to the molar mass of the substance expressed in grams per mole.

You will need
- - periodic table of chemical elements;
- - sealed container;
- - scales;
- - pressure gauge;
- - thermometer.
Instructions
If a substance is known, determine its molecular mass using Mendeleev’s periodic table of chemical elements. To do this, determine the elements that are in the formula of the substance. Then, find their relative atomic masses, which are written in the table. If the atomic mass in the table is represented as a fraction, round it to the nearest whole number. If it contains several atoms of a given element, multiply the mass of one atom by their number. Add the resulting atomic masses and get the relative molecular mass of the substance.
For example, to find the molecular weight of sulfuric H2SO4, find the relative atomic masses of the elements that are included in the formula, respectively, sulfur and oxygen Ar(H)=1, Ar(S)=32, Ar(O)=16. Considering that there are 2 atoms of hydrogen in a molecule, and 4 atoms of oxygen, calculate the molecular mass of the substance Mr(H2SO4)=2 1+32+4∙16=98 atomic mass units.
If you know the amount of a substance in moles ν and the mass of the substance m, expressed in grams, determine its molar mass; for this, divide the mass by the amount of the substance M=m/ν. It will be numerically equal to its relative molecular weight.
If the number of molecules of a substance N and mass m are known, find its molar mass. It will be equal to the molecular mass by finding the ratio of the mass in grams to the number of molecules of the substance in this mass, and multiply the result by Avogadro’s constant NА=6.022^23 1/mol (M=m∙N/NA).
To find the molecular mass of an unknown gas, find its mass in a sealed container of known volume. To do this, pump the gas out of it, creating a vacuum there. Weigh it. Then pump the gas back in and find its mass again. The difference in mass of the empty and inflated cylinder will be equal to the mass of the gas. Measure the pressure inside the cylinder using a pressure gauge in Pascals and in Kelvins. To do this, measure the ambient air temperature, it will be equal inside the cylinder in degrees Celsius, to convert it to Kelvin, add 273 to the resulting value.
Determine the molar mass of the gas by finding the product of temperature T, gas mass m and the universal gas constant R (8.31). Divide the resulting number by the values of pressure P and volume V, measured in m³ (M=m 8.31 T/(P V)). This number will correspond to the molecular weight of the gas being tested.
Hydrogen is the first element of the periodic table and the most common in the Universe, since it is what stars are mainly made of. It is part of a substance vital for biological life - water. Hydrogen, like any other chemical element, has specific characteristics, including molar mass.

Instructions
Remember, molar mass? This is the mass of one mole, that is, the amount that contains approximately 6.022 * 10^23 elementary particles of matter (atoms, molecules, ions). This number is called the “Avogadro number”, and is named after the famous scientist Amedeo Avogadro. The molar mass of a substance is numerically the same as its molecular mass, but has a different dimension: not atomic mass units (amu), but gram/mol. Knowing this, determine the molar mass hydrogen as easy as pie.
What kind of molecule does it have? hydrogen? It is diatomic, with the formula H2. Immediately: a molecule is considered consisting of two atoms of the lightest and most common hydrogen isotope, protium, and not of the heavier
The relative molecular weight of a substance shows how many times a molecule of a given substance is heavier than 1/12 of a pure carbon atom. It can be found if its chemical formula is known using Mendeleev's periodic table of elements. Otherwise, use other methods to find molecular mass, keeping in mind that it is numerically equal to the molar mass of the substance expressed in grams per mole.
You will need
- - periodic table of chemical elements;
- - sealed container;
- - scales;
- - pressure gauge;
- - thermometer.
Instructions
- If the chemical formula of a substance is known, determine its molecular mass using Mendeleev’s periodic table of chemical elements. To do this, determine the elements that are included in the formula of the substance. Then, find their relative atomic masses, which are written in the table. If the atomic mass in the table is represented as a fraction, round it to the nearest whole number. If a chemical formula contains several atoms of a given element, multiply the mass of one atom by their number. Add the resulting atomic masses and get the relative molecular mass of the substance.
- For example, to find the molecular weight of sulfuric acid H2SO4, find the relative atomic masses of the elements that are included in the formula, respectively, hydrogen, sulfur and oxygen Ar(H)=1, Ar(S)=32, Ar(O)=16. Considering that there are 2 atoms of hydrogen in a molecule, and 4 atoms of oxygen, calculate the molecular mass of the substance Mr(H2SO4)=2 1+32+4∙16=98 atomic mass units.
- If you know the amount of a substance in moles ν and the mass of the substance m, expressed in grams, determine its molar mass; for this, divide the mass by the amount of the substance M=m/ν. It will be numerically equal to its relative molecular weight.
- If the number of molecules of a substance N and mass m are known, find its molar mass. It will be equal to the molecular mass by finding the ratio of the mass in grams to the number of molecules of the substance in this mass, and multiply the result by Avogadro’s constant NА=6.022^23 1/mol (M=m∙N/NA).
- To find the molecular mass of an unknown gas, find its mass in a sealed container of known volume. To do this, pump the gas out of it, creating a vacuum there. Weigh the cylinder. Then pump the gas back in and find its mass again. The difference in mass of the empty and inflated cylinder will be equal to the mass of the gas. Measure the pressure inside the cylinder using a pressure gauge in Pascals and the temperature in Kelvin. To do this, measure the temperature of the surrounding air, it will be equal to the temperature inside the cylinder in degrees Celsius, to convert it to Kelvin, add 273 to the resulting value. Determine the molar mass of the gas by finding the product of temperature T, gas mass m and the universal gas constant R (8, 31). Divide the resulting number by the values of pressure P and volume V, measured in m³ (M=m 8.31 T/(P V)). This number will correspond to the molecular weight of the gas being tested.
Molecular mass- one of the most important characteristics of a substance. This concept is closely related to the definition of a molecule.
For conditional structural particles (formula units) of non-molecular substances, the concept of “formula mass” is used.
The masses of structural units of matter are very small. Therefore, relative masses are used for them.
Relative molecular weight is denoted by M r .
The relative formula mass of non-molecular substances is also denoted M r .
The values of relative molecular masses are widely used in various chemical, physical and chemical-technical calculations. Therefore, it is important to be able to calculate them.
Calculation of the relative molecular mass of a substance from its chemical formula. Using the chemical formula of a substance, you can not only characterize its composition, but also calculate the relative molecular mass or (for non-molecular compounds) the relative formula mass.
Relative molecular weight consists of the relative masses of the atoms that enter the molecule, taking into account their number.
Example. Let's calculate relative molecular weight of sulfuric acid H 2 SO 4 (Fig. 9.1). The relative mass of a sulfuric acid molecule consists of the sum of the relative masses of two hydrogen atoms, one sulfur atom and four oxygen atoms:
M r (H 2 SO 4) = 2A r (H) + A r (S) + 4A r (0);
M r (H 2 SO 4) = 2. 1 + 32 + 4 . 16 = 98.
The relative formula masses of non-molecular substances are calculated in the same way.
Example. Let's calculate relative formula mass of calcium fluoride CaF 2 (Fig. 9.2). The relative mass of the formula unit of calcium fluoride consists of the sum of the relative masses of the calcium cation Ca 2+ and two fluoride anions F -:
M r (CaF 2) = A r (Ca) + 2A r (F); M r (CaF 2) = 40 + 2. 19 = 78.
 |
| Rice. 9.3. Composition and structure of uvarovite |
Often chemical formulas of substances contain parentheses. For example, the composition of the mineral uvarovite is described by the chemical formula Ca 3 Cr 2 (SiO 4) 3. Uvarovite is an ionic crystal (Fig. 9.3). The relative mass of its formula unit can be calculated as follows: Material from the site
M r (Ca 3 Cr 2 (SiO 4) 3) = 3A r (Ca) + 2A r (Cr) + 3;
M r (Ca 3 Cr 2 (SiO 4) 3) = 3. 40 + 2. 52 + 3(28 + 4.16) = 500.
Please note: the indices that appear in the chemical formula outside the brackets are pronounced like this - twice, three times, etc.
The composition of the Coca-Cola drink includes water H 2O, carbon dioxide WITHO 2, coal H 2 CO 3 and phosphorus H 3 RO 4 acids, sucrose (sugar) C 12 H 22O 11, caffeineC 8H 10N 4O2.
On this page there is material on the following topics:
Atoms and molecules are the smallest particles of matter, so you can choose the mass of one of the atoms as a unit of measurement and express the masses of other atoms in relation to the chosen one. So what is molar mass, and what is its dimension?
What is molar mass?
The founder of the theory of atomic masses was the scientist Dalton, who compiled a table of atomic masses and took the mass of the hydrogen atom as one.
Molar mass is the mass of one mole of a substance. A mole, in turn, is an amount of substance that contains a certain number of tiny particles that participate in chemical processes. The number of molecules contained in one mole is called Avogadro's number. This value is constant and does not change.

Rice. 1. Formula for Avogadro's number.
Thus, the molar mass of a substance is the mass of one mole, which contains 6.02 * 10^23 elementary particles.
Avogadro's number got its name in honor of the Italian scientist Amedeo Avagadro, who proved that the number of molecules in equal volumes of gases is always the same
Molar mass in International system SI is measured in kg/mol, although this value is usually expressed in grams/mol. This value is designated English letter M, and the molar mass formula is as follows:
where m is the mass of the substance, and v is the amount of the substance.

Rice. 2. Calculation of molar mass.
How to find the molar mass of a substance?
The table of D.I. Mendeleev will help you calculate the molar mass of a particular substance. Let's take any substance, for example, sulfuric acid. Its formula is as follows: H 2 SO 4. Now let's turn to the table and see what the atomic mass of each of the elements included in the acid is. Sulfuric acid consists of three elements - hydrogen, sulfur, oxygen. The atomic mass of these elements is respectively 1, 32, 16.
It turns out that the total molecular mass is equal to 98 atomic mass units (1*2+32+16*4). Thus, we found out that one mole of sulfuric acid weighs 98 grams.
The molar mass of a substance is numerically equal to the relative molecular mass if the structural units of the substance are molecules. The molar mass of a substance can also be equal to the relative atomic mass if the structural units of the substance are atoms.
Until 1961, an oxygen atom was taken as an atomic mass unit, but not a whole atom, but 1/16 of it. At the same time, the chemical and physical units of mass were not the same. Chemical was 0.03% more than physical.
Currently, a unified measurement system has been adopted in physics and chemistry. As standard e.a.m. 1/12 of the mass of a carbon atom is selected.

Rice. 3. Formula for the unit of atomic mass of carbon.
The molar mass of any gas or vapor is very easy to measure. It is enough to use control. The same volume of a gaseous substance is equal in amount to another at the same temperature. A well-known way to measure the volume of steam is to determine the amount of displaced air. This process is carried out using a side branch leading to a measuring device.
The concept of molar mass is very important for chemistry. Its calculation is necessary for the creation of polymer complexes and many other reactions. In pharmaceuticals, the concentration of a given substance in a substance is determined using molar mass. Also, molar mass is important when conducting biochemical research (the metabolic process in an element).
Nowadays, thanks to the development of science, the molecular masses of almost all components of blood, including hemoglobin, are known.
Instructions
The unit of molecular mass is 1/12 of the mass of an atom, which is conventionally taken to be 12. Molecular mass is the total relative atomic mass of all the atoms in a molecule, and it is very easy to calculate.
And there is the simplest option if you know the substances. Take the periodic table, look at the molecular weight of each element included in. For example, for hydrogen it is equal to 1. – 16. And to find the molecular mass of the entire substance (take for example water, which consists of two hydrogen molecules and one), simply add up the masses of all the elements included in it. For water: M(H2O) = 2M(H)+M(O) = 2 1+16 = 18 a. eat.
Helpful advice
As you can see, finding the molecular weight is very simple. The main thing is not to confuse it with the molar mass of a substance - they are numerically equal to each other, but have different units of measurement and physical meaning.
Sources:
- Determine the molecular formula of a hydrocarbon if
Video on the topic
Sources:
- Experience as a teacher
In order to determine mass atom, find the molar mass of a monatomic substance using the periodic table. Then divide this mass by Avogadro's number (6.022 10^(23)). This will be the mass of the atom, in the units in which the molar mass was measured. The mass of a gas atom is found through its volume, which is easy to measure.

You will need
- To determine the mass of an atom of a substance, take the periodic table, a tape measure or ruler, a pressure gauge, or a thermometer.
Instructions
Determination of atomic mass solid or To determine the mass of an atom of a substance, determine it (what it consists of). In the periodic table, find the cell that describes the corresponding element. Find the mass of one mole of this substance in grams per mole that is in this cell (this number corresponds to the mass of the atom in atomic mass units). Divide the molar mass of the substance by 6.022 10^(23) (Avogadro's number), the result will be the substance in grams. You can determine the mass of an atom in another way. To do this, multiply the atomic mass of the substance in atomic mass units taken from the periodic table by the number 1.66 10^(-24). Get the mass of one atom in grams.
Determining the mass of a gas atom If there is an unknown gas in the vessel, determine its mass in grams by weighing the empty vessel and the vessel with the gas, and find the difference in their masses. After this, measure the volume of the vessel using a ruler or tape measure, followed by calculations or other methods. Express the result in . Use a pressure gauge to measure the gas pressure inside the vessel, and measure its temperature with a thermometer. If the thermometer scale is graduated in Celsius, determine the temperature in Kelvin. To do this, add the number 273 to the temperature value on the thermometer scale.
Determining the molar mass of a substance from the mass of a molecule If the mass of one molecule in grams is known, multiply it by Avogadro's number 6.022 10^(23), which is equal to the number of molecules in one mole of the substance. The result will be substances in grams per mole. Having found it in the periodic table, if necessary, determine the substance itself, if it is simple (consists of a monatomic molecule).
Determining the molar mass of a gas Take a vessel of known volume and put a certain mass of gas into it. To do this, first pump the gas out of it and weigh it, and then pump the gas in and weigh it again. After this, measure the gas pressure in pascals using a thermometer and its temperature. To convert Celsius to , add 273 to them. In order to find the molar mass, transforming the Clapeyron-Mendeleev equation, take the value of the gas mass in grams, multiply it by the temperature and the number 8.31, which is universal. Sequentially divide the resulting number by the pressure in cubic meters (M=m 8.31 T/(P V)). The result will be the molar mass of the gas in grams per mole.
Video on the topic
Sources:
- molar masses of substances table
In order to find the molar mass substances, determine its chemical formula and, using the periodic table of Mendeleev, calculate its molecular mass. It is numerically equal to the molar mass substances in grams per mole. If the mass of one molecule is known substances, convert it to grams and multiply by 6.022 10^23 (Avogadro's number). Molar mass gas can be found using the ideal gas equation of state.

You will need
- Periodic table, pressure gauge, thermometer, scales.
Instructions
Determination of molar mass using a chemical formula. Find the elements in the periodic table that correspond to the atoms the molecule consists of substances. If a molecule substances monoatomic, then this will be his. If not, find the atomic mass of each element and add up these masses. The result will be molar mass substances, expressed in grams per mole.
Determination of molar mass substances by mass of one molecule. If the mass of one molecule is known, convert it to , then multiply by the number of molecules in one mole of any substances, which is 6.022 10^23 (Avogadro's number). Get molar mass substances in grams per mole.
Determination of the molar mass of a gas. Take a cylinder that can be hermetically sealed with a predetermined volume, which is converted to . Using a pump, pump out the gas from it and weigh the empty cylinder on the scale. Then fill it with a gas whose molar mass is measured. Weigh the cylinder again. The difference in the masses of an empty and filled with gas cylinder will be the mass of gas, express it in grams.
Using a pressure gauge, measure the gas pressure inside the cylinder; to do this, attach it to the gas injection hole. You can immediately use a cylinder with a built-in pressure gauge to quickly monitor pressure readings. Measure pressure in pascals.
Wait a while for the gas inside the cylinder to reach the temperature environment, and measure it with a thermometer. Convert the indicator from degrees Celsius to kelvins by adding the number 273 to the measured value.
Multiply the gas mass by the temperature and the universal gas constant (8.31). Sequentially divide the resulting number into pressure and volume values (M=m 8.31 T/(P V)). The result will be the molar mass of the gas in grams per mole.
Sources:
- determination of molar mass
Molecular weight is the molecular weight, which can also be called the mass value of a molecule. Molecular mass is expressed in atomic mass units. If we analyze the value of the molecular mass in parts, it turns out that the sum of the masses of all the atoms that make up the molecule represents its molecular mass mass. If we talk about units of measurement of mass, then predominantly all measurements are made in grams.

Instructions
Molecular mass itself is associated with the concept of a molecule. But it cannot be said that this condition can only be applied to those where the molecule, for example, hydrogen, is located separately. For cases where the molecules are not separately from the rest, but in close interconnection, all of the above conditions and definitions are also valid.
To begin with, to determine mass hydrogen, you will need -, which contains hydrogen and from which it can be easily isolated. This can be some kind of alcohol solution or other mixture, some of the components of which, under certain conditions, change their state and easily free the solution from its presence. Find a solution from which you can evaporate necessary or unnecessary substances using heat. This is the most easy way. Now decide whether you will evaporate a substance that you do not need or whether it will be hydrogen, a molecular mass which you plan to measure. If an unnecessary substance evaporates, it’s okay, so long as it’s not toxic. in the case of evaporation of the desired substance, you need equipment so that all the evaporation is preserved in the flask.
After you have separated everything unnecessary from the composition, start measuring. For this purpose, Avogadro's number is suitable for you. It is with its help that you can calculate the relative atomic and molecular mass hydrogen. Find all the options you need hydrogen which are present in any table, determine the density of the resulting gas, as it will be useful for one of the formulas. Then substitute all the results obtained and, if necessary, change the unit of measurement to , as mentioned above.
The concept of molecular weight is most relevant when it comes to polymers. It is for them that it is more important to introduce the concept of average molecular weight, due to the heterogeneity of the molecules included in their composition. Also, by the average molecular weight, one can judge how high the degree of polymerization of a particular substance is.
Video on the topic
Molecular mass is the mass of a molecule of a substance, expressed in atomic units. The task often arises is to determine the molecular weight. How can I do that?

Instructions
If you know, then the problem can be solved in an elementary way. All you need is the Periodic Table. For example, you need to find the molecular weight of chloride. Write the formula of the substance: CaCl2. Using the periodic table, determine the atomic mass of each element included in its composition. For calcium it is equal to (rounded) 40, for (also rounded) – 35.5. Taking into account index 2, find: 40 + 35.5*2 = 111 a.m.u. (atomic mass units).
But what about in cases where the exact substance is unknown? You can act here in different ways. One of the most effective (and at the same time simple) is the so-called “osmotic pressure method”. It is based on osmosis, which consists in the fact that solvent molecules can penetrate through a semi-permeable membrane, while solute molecules cannot penetrate through it. The magnitude of osmotic pressure can be measured, and it is directly proportional to the concentration of molecules of the substance under study (that is, their number per unit volume of solution).
Some people are familiar with the universal Mendeleev-Clapeyron equation, which describes the state of the so-called “ideal gas”. It looks like this: PVm = MRT. Van't Hoff's formula is very similar: P = CRT, where P is the osmotic pressure, C is the molar concentration of the solute, R is the universal gas constant, T is the temperature in Kelvin. This similarity is not accidental. It was as a result of Van't Hoff's work that it became clear that molecules (or ions) behave as if they were in a gas (with the same volume).
By measuring the osmotic pressure, you can simply calculate the molar concentration: C=P/RT. And then, knowing also the mass of the substance in the solution, find its molecular mass. Suppose it was experimentally established that the molar concentration of the already mentioned substance is 0.2. Moreover, the solution contains 22.2 grams of this substance. What is its molecular weight? 22.2/0.2 = 111 amu - exactly the same as the previously mentioned calcium chloride.
Video on the topic
Molecular mass substances is the mass of a molecule, expressed in atomic units and numerically equal to the molar mass. When calculating in chemistry, physics and technology, the calculation of the molar mass of various substances is often used.

You will need
- - Mendeleev table;
- - table of molecular weights;
- - table of cryoscopic constant values.
Instructions
Find the required element in the periodic table. Pay attention to the fractional numbers under its sign. For example, O has a numerical value in the cell equal to 15.9994. This is the atomic mass of the element. Atomic mass must be multiplied by the index of the element. The index shows how much of an element is contained in a substance.
If given complex, then multiply the atomic mass each element by its index (if there is one atom of a particular element and there is no index, then multiply by one) and add the resulting atomic masses. For example, water is calculated as follows - MH2O = 2 MH + MO ≈ 2·1+16 = 18 a. eat.
Calculate molar mass using suitable formulas and equate it to molecular. Change the units of measurement from g/mol to amu. If pressure, volume, absolute Kelvin temperature and mass are given, calculate the molar mass gas according to the Mendeleev-Cliperon equation M=(m∙R∙T)/(P∙V), in which M is the molecular () in amu, R is the universal gas constant.
Calculate molar mass according to the formula M=m/n, where m is the mass of any given substances, n - chemical quantity substances. Express the quantity substances through Avogadro's number n=N/NA or using volume n=V/VM. Substitute into the formula above.
Find the molecular mass gas, if only the value of its volume is given. To do this, take a sealed cylinder of known volume and pump out
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0