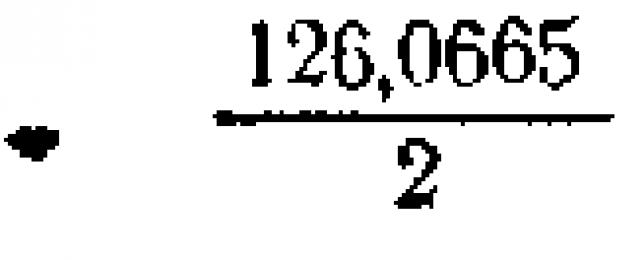approximate solutions. The most common alkali solutions in laboratory practice are solutions of caustic soda NaOH. Solutions of caustic potash KOH are rarely prepared, but ammonia solutions are almost always bought ready-made.
Caustic soda (or caustic potash) is commercially available in the form of preparations: technical, pure and chemically pure. The difference between them is the percentage of NaOH (or KOH)1 and, consequently, impurities. Technical * NaOH contains significant amounts of NaCl, Na2CO3, Na2SiO3, Fe2O3, etc. A pure reagent contains a minimum amount of these impurities, and a chemically pure reagent contains only traces of them.
Technical caustic soda is sold cast in iron barrels, pure - in lamellar pieces, and chemically pure - in the form of sticks or tablets.
When alkali is dissolved, strong heating occurs, especially in those places where pieces of it lie. To make the dissolution go faster, the solution should be stirred all the time with a glass rod.
It is not recommended to use glassware when dissolving alkali, because it can easily break and the worker can be injured, since a concentrated solution of alkali corrodes skin, shoes and clothes. If you have to prepare small amounts of alkali, then you can dissolve it in glassware.
Pieces of alkali cannot be taken with bare hands, they should be taken with crucible tongs, special tweezers or, in extreme cases, with hands, but always with rubber gloves.
* In engineering, caustic soda is often called caustic soda.
alkalis, many impurities do not dissolve and, when the solution is settled, settle to the bottom. The settling of a concentrated alkali solution lasts several days (at least two) *. The settled solution is carefully drained, preferably by a siphon, into another vessel, and the precipitate is discarded or used for washing dishes.
If in the laboratory it is often necessary to prepare alkali solutions in large quantities, then the following method is used. First, the alkali is completely dissolved in a porcelain cup, and when the solution cools down a bit (to 40-50 ° C), it is poured through a funnel into a glass bottle of a suitable container. The bottle is well closed with a rubber stopper provided with a hole into which a calcium chloride tube filled with soda lime (to absorb carbon dioxide) is inserted. When the alkali settles and a sharply delimited layer of sediment forms at the bottom (1-2 cm from the bottom), the top layer of the solution is poured into another bottle. Two tubes are inserted into the rubber stopper of the latter, one of which should enter approximately 1/3 of the height of the bottle, and the other should be 1-2 cm below the stopper (Fig. 350).
A rubber tube with a glass end is placed on the outer end of a long glass tube, which is lowered into a bottle with settled alkali. The lower end of this tube should be bent as shown in Fig. 350. Such an end prevents the sediment from being captured from the bottom of the bottle, even if the end of the tube touches the sediment. A short tube is connected to a vacuum pump. Turning on the pump, the settled solution is quickly and safely pumped into another bottle. When transfusing alkali, care must be taken to ensure that the tube lowered into the vessel with settled alkali does not raise sediment from the bottom. Therefore, at the beginning of the transfusion, it is kept high enough above the sediment, gradually lowering towards the end of the transfusion.
After that, the density of the solution is determined with a hydrometer and the percentage of alkali is found from the table. If it is necessary to prepare a more dilute solution, then the dilution is carried out using the calculation methods described above.
* Naturally, the caustic soda solution must be settled without access to carbon dioxide. Strong alkali solutions strongly leach the glass of bottles, so the inside of the bottle must be coated with paraffin or a mixture of ceresin and vaseline, or an alloy of paraffin with polyethylene (see Ch. 3 "Clocks and their handling").
To cover the walls of the bottle with paraffin, several pieces of it are placed inside the bottle and the latter is heated in an oven or over an electric stove or gas burner (carefully) to 60-8O0C. When the paraffin is melted, the bottle is turned and the melted mass is distributed in a thin layer over the entire inner surface.
A paraffin or ceresin layer can be applied by using a solution of these substances in aviation gasoline. Paraffin is first dissolved in gasoline, the resulting solution is poured into a bottle, which must be covered inside with paraffin. The walls of the bottle are washed with the introduced paraffin solution, slowly turning it about the axis in a horizontal position. When a paraffin film forms on the glass, the bottle is blown with air until the gasoline vapors are completely expelled. Then the bottle is rinsed once or twice with water. Only after that it can be filled with alkali or other liquid.
Treatment of alkali storage bottles is especially important for analytical laboratories, as it prevents contamination of titrated solutions by glass leaching products.
precise solutions. The preparation of exact solutions differs in that they take chemically pure alkali, dissolve it as indicated above, and determine the alkali content by titration with an exact acid solution.
The titer of the tickling solution (i.e., the exact concentration of the solution) is best determined by a solution of oxalic acid (C2H2O4 2H2O)*.
Sales oxalic acid should be recrystallized once or twice and only then used to prepare an accurate solution. It is a dibasic acid and, therefore, its equivalent weight is half the molecular weight. Since the latter is equal to 126.0665, then its equivalent weight will be:

Cooking 0.1 and. NaOH solution, we must have a solution of oxalic acid of the same normality, for which it must be taken for 1 liter of solution:

But to set the titer, such an amount of solution is not needed; it is enough to prepare 100 ml or a maximum of 250 ml. For this, about 0.63 g (for 100 ml) of recrystallized oxalic acid is weighed on an analytical balance to the fourth decimal place.
Novice workers, when taking test portions for setting the titer, often try to weigh out the exact amount of the substance indicated in the manual (in our case, 0.6303 g). In no case should this be done, since such weighing inevitably requires repeated
* Since caustic soda easily absorbs carbon dioxide, sodium carbonate is always present in alkali.. Having prepared a solution of caustic soda, its concentration must be determined by titrating solutions of accurate weights of organic acids, such as oxalic, malic, etc. Therefore, there is no need to dilute the concentrated solution in a volumetric flask with bringing the level of the solution exactly to the mark; you can pour it into the bottle where it will be stored, add water with a graduated cylinder. It should be borne in mind that when preparing solutions of caustic alkalis, the main attention should be paid to protecting the solutions from carbon dioxide in the air. Any reduction in operations in which the solution may come into contact with air is highly desirable, pouring and pouring the substance into the container. As a result, part of the substance falls on the scales and on the outer wall of the container, and the accurately weighed amount of the substance cannot be completely transferred to the volumetric flask. Therefore, the prepared solution will be inaccurate. Finally, very many substances change in the air (lose water of crystallization or, as they say, “weather”, absorb carbon dioxide from the air, etc.). Therefore, the longer the weighing continues; the greater the potential for contamination. Therefore, first, a sample is taken on a technochemical balance that converges with the required one in the first two decimal places, and then the exact mass is determined on an analytical balance. The sample is dissolved in the appropriate volume of solvent.
Knowing the mass of the substance taken and the volume of the solution, it is easy to calculate its exact concentration, which in our case will not be equal to 0.1 N, but slightly less. With this method, the calculation is somewhat more complicated, but greater accuracy and significant time savings are achieved.
When the solution is ready, take 20 ml from it with a pipette, transfer it to a conical flask, add a few drops of phenolphthalein and titrate with the prepared alkali solution until a faint pink color appears.
Example. 22.05 ml of alkali solution was used for titration. Calculate its titer and normality.
Oxalic acid was taken 0.6223 g instead of the theoretically calculated amount of 0.6303 g. Therefore, the concentration of its solution is not exactly 0.1 N, but is equal to

To calculate the normality of an alkali solution, one should use the relation N
Interaction with acids
Alkalis, like bases, react with acids to form salt and water (neutralization reaction). This is one of the most important chemical properties of alkalis.
Alkali + Acid → Salt + Water
; .
Interaction with acid oxides
Alkalis interact with acidic oxides to form salt and water:
Alkali + Acid oxide → Salt + Water
;
Interaction with amphoteric oxides
.Interaction with transition metals
Alkali solutions react with metals, which form amphoteric oxides and hydroxides ( and etc). The equations of these reactions in a simplified form can be written as follows:
; .
In reality, in the course of these reactions, hydroxo complexes are formed in solutions (hydration products of the above salts):
; ;
Interaction with salt solutions
Alkali solutions interact with salt solutions if an insoluble base or insoluble salt is formed:
Alkali solution + Salt solution → New base + New salt
; ;
Receipt
Soluble bases are obtained in various ways.
Hydrolysis of alkali/alkaline earth metals
Obtained by electrolysis of alkali metal chlorides or by the action of water on alkali metal oxides.
Application
Alkalis are widely used in various industries and medicine; also for disinfection of ponds in fish farming and as a fertilizer, as an electrolyte for alkaline batteries.
Write a review on the article "Alkalis"
Notes
Literature
- Kolotov S.S.,.// Encyclopedic Dictionary of Brockhaus and Efron: in 86 volumes (82 volumes and 4 additional). - St. Petersburg. , 1890-1907.
- Glossary of terms in chemistry // J. Opeida, O. Schweika. Institute of Physical and Organic Chemistry and Coal Chemistry im. L.M. Litvinenka National Academy of Sciences of Ukraine, Donetsk National University - Donetsk: "Weber", 2008. - 758 p. - ISBN 978-966-335-206-0
An excerpt characterizing alkalis
- Here. What lightning! they were talking.In the abandoned tavern, in front of which stood the doctor's wagon, there were already about five officers. Marya Genrikhovna, a plump blond German woman in a blouse and nightcap, was sitting in the front corner on a wide bench. Her husband, the doctor, slept behind her. Rostov and Ilyin, greeted with cheerful exclamations and laughter, entered the room.
- AND! what fun you have, ”said Rostov, laughing.
- And what are you yawning?
- Good! So it flows from them! Don't wet our living room.
“Don’t get Marya Genrikhovna’s dress dirty,” the voices answered.
Rostov and Ilyin hurried to find a corner where, without violating the modesty of Marya Genrikhovna, they could change their wet clothes. They went behind the partition to change their clothes; but in a small closet, filling it all up, with one candle on an empty box, three officers were sitting, playing cards, and would not give up their place for anything. Marya Genrikhovna gave up her skirt for a while in order to use it instead of a curtain, and behind this curtain, Rostov and Ilyin, with the help of Lavrushka, who brought packs, took off their wet and put on a dry dress.
A fire was kindled in the broken stove. They took out a board and, having fixed it on two saddles, covered it with a blanket, took out a samovar, a cellar and half a bottle of rum, and, asking Marya Genrikhovna to be the hostess, everyone crowded around her. Who offered her a clean handkerchief to wipe her lovely hands, who put a Hungarian coat under her legs so that it would not be damp, who curtained the window with a raincoat so that it would not blow, who fanned the flies from her husband’s face so that he would not wake up.
“Leave him alone,” said Marya Genrikhovna, smiling timidly and happily, “he sleeps well after a sleepless night.
“It’s impossible, Marya Genrikhovna,” answered the officer, “we must serve the doctor.” Everything, maybe, and he will take pity on me when he cuts his leg or arm.
There were only three glasses; the water was so dirty that it was impossible to decide when the tea was strong or weak, and there was only six glasses of water in the samovar, but it was all the more pleasant, in turn and seniority, to receive your glass from Marya Genrikhovna’s plump hands with short, not quite clean nails . All the officers really seemed to be in love with Marya Genrikhovna that evening. Even those officers who were playing cards behind the partition soon gave up the game and went over to the samovar, obeying the general mood of wooing Marya Genrikhovna. Marya Genrikhovna, seeing herself surrounded by such brilliant and courteous youth, beamed with happiness, no matter how hard she tried to hide it and no matter how obviously shy at every sleepy movement of her husband sleeping behind her.
There was only one spoon, there was most of the sugar, but they did not have time to stir it, and therefore it was decided that she would stir the sugar in turn for everyone. Rostov, having received his glass and poured rum into it, asked Marya Genrikhovna to stir it.
- Are you without sugar? she said, smiling all the time, as if everything she said, and everything others said, was very funny and had another meaning.
- Yes, I don’t need sugar, I just want you to stir with your pen.
Marya Genrikhovna agreed and began to look for the spoon, which someone had already seized.
- You're a finger, Marya Genrikhovna, - said Rostov, - it will be even more pleasant.
- Hot! said Marya Genrikhovna, blushing with pleasure.
Ilyin took a bucket of water and, dropping rum into it, came to Marya Genrikhovna, asking her to stir it with her finger.
“This is my cup,” he said. - Just put your finger in, I'll drink it all.
When the samovar was all drunk, Rostov took the cards and offered to play kings with Marya Genrikhovna. A lot was cast as to who should form the party of Marya Genrikhovna. The rules of the game, at the suggestion of Rostov, were that the one who would be the king had the right to kiss the hand of Marya Genrikhovna, and that the one who remained a scoundrel would go to put a new samovar for the doctor when he wakes up.
“Well, what if Marya Genrikhovna becomes king?” Ilyin asked.
- She's a queen! And her orders are the law.
The game had just begun, when the doctor's confused head suddenly rose from behind Marya Genrikhovna. He had not slept for a long time and listened to what was said, and apparently did not find anything cheerful, funny or amusing in everything that was said and done. His face was sad and dejected. He did not greet the officers, scratched himself and asked for permission to leave, as he was blocked from the road. As soon as he left, all the officers burst into loud laughter, and Marya Genrikhovna blushed to tears, and thus became even more attractive to the eyes of all the officers. Returning from the yard, the doctor told his wife (who had already ceased to smile so happily and, fearfully awaiting the verdict, looked at him) that the rain had passed and that we had to go to spend the night in a wagon, otherwise they would all be dragged away.
- Yes, I'll send a messenger ... two! Rostov said. - Come on, doctor.
"I'll be on my own!" Ilyin said.
“No, gentlemen, you slept well, but I haven’t slept for two nights,” said the doctor, and sat down gloomily beside his wife, waiting for the game to be over.
Looking at the gloomy face of the doctor, looking askance at his wife, the officers became even more cheerful, and many could not help laughing, for which they hastily tried to find plausible pretexts. When the doctor left, taking his wife away, and got into the wagon with her, the officers lay down in the tavern, covering themselves with wet overcoats; but they didn’t sleep for a long time, now talking, remembering the doctor’s fright and the doctor’s merriment, now running out onto the porch and reporting what was happening in the wagon. Several times Rostov, wrapping himself up, wanted to fall asleep; but again someone's remark amused him, again the conversation began, and again there was heard the causeless, cheerful, childish laughter.
At three o'clock, no one had yet fallen asleep, when the sergeant-major appeared with the order to march to the town of Ostrovna.
All with the same accent and laughter, the officers hurriedly began to gather; again put the samovar on the dirty water. But Rostov, without waiting for tea, went to the squadron. It was already light; The rain stopped, the clouds dispersed. It was damp and cold, especially in a damp dress. Leaving the tavern, Rostov and Ilyin both looked in the twilight of dawn into the doctor's leather tent, glossy from the rain, from under the apron of which the doctor's legs stuck out and in the middle of which the doctor's bonnet was visible on the pillow and sleepy breathing was heard.
"Really, she's very nice!" Rostov said to Ilyin, who was leaving with him.
- What a lovely woman! Ilyin replied with sixteen-year-old seriousness.
Half an hour later, the lined up squadron stood on the road. The command was heard: “Sit down! The soldiers crossed themselves and began to sit down. Rostov, riding forward, commanded: “March! - and, stretching out in four people, the hussars, sounding with the slapping of hooves on the wet road, the strumming of sabers and in a low voice, set off along the large road lined with birches, following the infantry and the battery walking ahead.
Broken blue-lilac clouds, reddening at sunrise, were quickly driven by the wind. It got brighter and brighter. One could clearly see that curly grass that always sits along country roads, still wet from yesterday's rain; the hanging branches of the birch trees, also wet, swayed in the wind and dropped light drops to the side. The faces of the soldiers became clearer and clearer. Rostov rode with Ilyin, who did not lag behind him, along the side of the road, between a double row of birches.
Rostov in the campaign allowed himself the freedom to ride not on a front-line horse, but on a Cossack. Both a connoisseur and a hunter, he recently got himself a dashing Don, large and kind playful horse, on which no one jumped him. Riding this horse was a pleasure for Rostov. He thought of the horse, of the morning, of the doctor's wife, and never once thought of the impending danger.
- In contact with 0
- Google Plus 0
- OK 0
- Facebook 0