Any substance in the world has certain magnetic properties. They are measured by magnetic permeability. In this article, we will consider the magnetic properties of matter.
Ampère hypothesis
Magnetic permeability shows how many times less or more magnetic field induction in a given medium of magnetic field induction in vacuum.
A substance that creates its own magnetic field is called magnetized. Magnetization occurs when a substance is placed in an external magnetic field.
The French scientist Ampère established the cause, the consequence of which is the possession of magnetic properties by bodies. Ampère's hypothesis says that inside the substance there are microscopic electric currents(an electron has its own magnetic moment, which has a quantum nature, orbital motion in electron atoms). It is they who determine the magnetic properties of matter. If the currents have disordered directions, then magnetic fields that they generate cancel each other out. The body is not magnetized. An external magnetic field regulates these currents. As a result, the substance has its own magnetic field. This is the magnetization of matter.
It is precisely by the reaction of substances to an external magnetic field and by the ordering of their internal structure, determine the magnetic properties of a substance. In accordance with these parameters, they are divided into the following groups:
- Paramagnets
- Diamagnets
- ferromagnets
- Antiferromagnets
Diamagnets and paramagnets
- Substances that have a negative magnetic susceptibility, independent of the strength of the magnetic field, are called diamagnets. Let's see what magnetic properties of a substance are called negative magnetic susceptibility. This is when a magnet is brought to the body, and at the same time it is repelled, not attracted. Diamagnets include, for example, inert gases, hydrogen, phosphorus, zinc, gold, nitrogen, silicon, bismuth, copper, silver. That is, these are substances that are in a superconducting state or have covalent bonds.
- Paramagnets. For these substances, the magnetic susceptibility also does not depend on what field strength exists. She is positive though. That is, when a paramagnet approaches a permanent magnet, an attractive force arises. These include aluminum, platinum, oxygen, manganese, iron.
ferromagnets
Substances with a high positive magnetic susceptibility are called ferromagnets. In these substances, in contrast to diamagnets and paramagnets, the magnetic susceptibility depends on temperature and magnetic field strength, and to a large extent. These include nickel and cobalt crystals.
Antiferromagnets and ferrimagnets
- Substances in which, during heating, a phase transition of a given substance occurs, accompanied by the appearance of paramagnetic properties, are called antiferromagnets. If the temperature becomes below a certain one, these properties of the substance will not be observed. Examples of these substances would be manganese and chromium.
- Ferrimagnets are characterized by the presence of uncompensated antiferromagnetism in them. Their magnetic susceptibility also depends on temperature and magnetic field strength. But they still have differences. These substances include various oxides.
All of the above magnets can be further divided into 2 categories:
- hard magnetic materials. These are materials from high value coercive force. For their magnetization reversal, it is necessary to create a powerful magnetic field. These materials are used in the manufacture of permanent magnets.
- Soft magnetic materials, on the contrary, have a small coercive force. In weak magnetic fields, they are able to enter saturation. They have low losses for magnetization reversal. Because of this, these materials are used to make cores for electrical machines that run on alternating current. This, for example, is a current and voltage transformer, or a generator, or an asynchronous motor.
We examined all the basic magnetic properties of matter and figured out what types of magnets exist.
3.5 Magnetic properties of matter
3.5.1 Magnetics. Magnetic properties of substances
In the previous chapter, it was assumed that the wires carrying currents that create a magnetic field are in a vacuum. If current-carrying wires are in any environment, the magnetic field changes. This is explained by the fact that any substance is a magnet, that is, it is capable of acquiring a magnetic moment (be magnetized) under the influence of a magnetic field. A magnetized substance creates a magnetic field AT " , which is superimposed on the current-induced field AT 0 . Both fields add up to the resulting field
|
AT = AT 0 + AT " |
This phenomenon was first discovered by Ampère, who found that adding an iron core to a solenoid is tantamount to increasing the number of ampere turns of that solenoid. Subsequently, it was found that the induction AT the magnetic field in a substance can be both larger and smaller than the induction B 0 the same field in vacuum. This happens because every substance, to a greater or lesser extent, has its own magnetic properties. AT ".
Substances capable of changing the parameters of a magnetic field are called magnets. To characterize the magnetic properties of substances, the quantity μ = B/ B 0 , called magnetic permeability this substance. According to the value of magnetic permeability, all magnets are divided into three groups.
a) Since the internal magnetic field in the diamagnet is directed against the external field, the induction modulus of the resulting field in the diamagnet is less than the field induction modulus in vacuum, i.e. AT<AT 0 . So substances that have μ<. l, called diamagnets. These include, for example, the elements Bi, Cu, Ag, Au, Hg, Be, CI, inert gases and other substances. Magnetic permeability μ diamagnet is independent of induction AT 0 external magnetic field.
b) Paramagnetic substances consist of atoms in which the orbital magnetic moments of electrons are not compensated. Therefore, the atoms of a diamagnet have non-zero magnetic moments. However, in the absence of an external magnetic field, the thermal motion of atoms leads to a chaotic arrangement of their magnetic moments, as a result of which any volume of a paramagnet as a whole does not have a magnetic moment.
When a paramagnet is introduced into an external magnetic field, its atoms to a greater or lesser extent (depending on the induction of this field) are arranged so that their magnetic moments are oriented in the direction of the external field. As a result, an internal magnetic field arises in the paramagnet, the induction of which B coincides in direction with the induction Bn of the external field. So the modulus of induction AT the resulting magnetic field in the paramagnet is greater than the modulus of induction AT 0 fields in vacuum, i.e. B>B 0 . So paramagnets called substances in which μ>1. These include, in particular, Na, Mg, K, Ca, Al, Mn, Pt, oxygen and many other elements, as well as solutions of some salts. Magnetic permeability μ paramagnets, like diamagnets, do not depend on induction AT 0 external magnetic field.
It should be noted that the value μ for dia- and paramagnets, it differs from unity very little, only by a value of the order of 10 -5 - 10 -6, therefore, dia- and paramagnets are classified as weakly magnetic substances.
c) Unlike dia- and paramagnets, in which the magnetic properties are determined by the orbital magnetic moments of atomic electrons, the magnetic properties of ferromagnets are due to the spin magnetic moments of the electrons. Ferromagnetic substances (always having a crystalline structure) are composed of atoms in which not all electrons have mutually canceled spin magnetic moments.
In a ferromagnet, there are regions of spontaneous ( spontaneous ) magnetizations, which are called domains. (The size of the domains is about 10 -4 - 10 -7 m.) In each domain, the spin magnetic moments of atomic electrons have the same orientation, as a result of which the domain is magnetized to a saturation state. Since, in the absence of an external magnetic field, the magnetic moments of the domains are randomly oriented, a ferromagnetic sample under such conditions is generally not magnetized.
Under the action of an external magnetic field, the magnetic moments of the domains are oriented along the direction of this field. As a result, a strong internal magnetic field arises in a ferromagnet with magnetic induction AT", coinciding in direction with the magnetic induction of the external field AT 0 . So the modulus of induction AT the resulting magnetic field in a ferromagnet is much larger, the field in vacuum, i.e. B»B 0 . When all the magnetic moments of the domains under the action of an external magnetic field are oriented along the field, saturation of the ferromagnetic sample occurs.
Upon reaching certain temperature points for each substance, called the Curie point above, the domain structure is destroyed, and the ferromagnet loses its inherent properties.
Thus, substances in which μ»1 are called ferromagnets. These include the elements Fe, Co, Ni, Gd and many alloys. In an external magnetic field, a ferromagnetic sample behaves like a paramagnet. However, the magnetic permeability μ of a ferromagnet depends on the intensity H external magnetic field and varies over a fairly wide range, as a result of which the dependence B =f(H) is non-linear . The values of μ for some alloys reach tens of thousands. Therefore, ferromagnets are classified as highly magnetic substances.
For every ferromagnet, there is a certain temperature, called Curie point, when heated above which the given substance loses its ferromagnetic properties and turns into a paramagnet. For example, for Fe, the Curie point is 1043 K, and for Ni it is 631 K.
To explain the process of magnetization of bodies, Ampère suggested that circular currents (molecular currents) circulate in the molecules of matter. Each such current has a magnetic moment and creates a magnetic field in the surrounding space. In the absence of an external field, the molecular currents are randomly oriented, as a result of which the resulting field due to them is zero. Due to the random orientation of the magnetic moments of individual molecules, the total magnetic moment of the body is also equal to zero. Under the action of the field, the magnetic moments of the molecules acquire a predominant orientation in one direction, as a result of which the magnet is magnetized - its total magnetic moment becomes different from zero. The magnetic fields of individual molecular currents in this case no longer compensate each other and a field arises AT". The magnetization of a magnet is naturally characterized by the magnetic moment per unit volume. This value is called magnetization and denoted by the letter J. Magnetization is usually associated not with magnetic induction, but with the field strength. It is assumed that at every point
In contrast to the dielectric susceptibility, which can only have positive values (polarization R in an isotropic dielectric is always directed along the field E), magnetic susceptibility χ is both positive and negative. Therefore, the magnetic permeability μ can be either greater or less than unity.
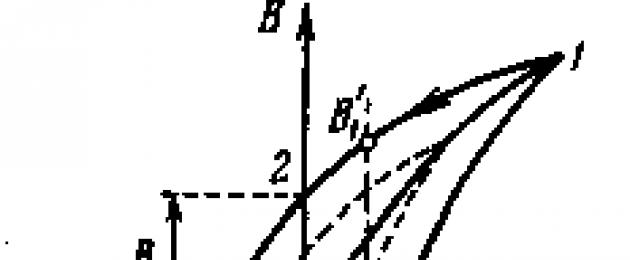
The magnetization of weakly magnetic substances varies linearly with the field strength. The magnetization of ferromagnets h, depends on H in a complicated way. In the figure - 3.39 dan magnetization curve ferromagnet, whose magnetic moment was initially zero. Already in fields on the order of several oersteds (~100 A/m), the magnetization J reaches saturation. The main magnetization curve in the diagram B - H shown in fig. 59.2 (curve 0-1). Upon reaching saturation AT continues to grow from H according to a linear law. If we bring the magnetization to saturation (point 1 in the figure - 3.40) and then reduce the magnetic field strength, then the induction AT does not follow the original curve 0-1, a changes according to the curve 1-2. As a result, when the strength of the external field becomes equal to zero (point 2), magnetization does not disappear and is characterized by the value AT r , which is called residual induction. Magnetization is important J r called remanent magnetization.
|
|
|
|
Figure - 3.39 |
Drawing - 3.40 |
Induction AT vanishes only under the influence of the field H with , having a direction opposite to the field that caused the magnetization. tension H with called coercive force.
The existence of residual magnetization makes it possible to manufacture permanent magnets, i.e., bodies that, without expending energy to maintain macroscopic currents, have a magnetic moment and create a magnetic field in their surroundings. A permanent magnet retains its properties the better, the greater the coercive force of the material from which it is made.
When an alternating magnetic field acts on a ferromagnet, the induction changes in accordance with the curve / - 2 -3-4-5-1 (Figure - 3.40), which is called hysteresis loop(a similar loop is obtained in the diagram J- H). If the maximum values H are such that the magnetization reaches saturation, the so-called maximum hysteresis loop is obtained (a solid loop in the figure is 3.40). If at amplitude values H saturation is not reached, a loop is obtained, called a partial loop (dotted loop in the figure). There are an infinite number of private cycles, they all lie inside the maximum hysteresis loop. Hysteresis leads to the fact that the magnetization of a ferromagnet is not a single-valued function H, it largely depended on the prehistory of the sample - on what fields it had been in before.
Due to the ambiguity of dependence AT from H the concept of magnetic permeability applies only to the main magnetization curve. Magnetic permeability of ferromagnets μ , therefore, the magnetic susceptibility χ is a function of the field strength. In the figure - 3.41 ,a the main magnetization curve is shown. (we draw a straight line from the origin of coordinates passing through an arbitrary point of the curve. The tangent of the angle of inclination: the straight line is proportional to the ratio V/N, t. e. magnetic permeability μ, for the corresponding tension value N. With an increase H from zero the angle of inclination (and hence μ ) first increases. At the point 2 it reaches a maximum (direct O is tangent to the curve) and then decreases. In the figure - 3.41, b dependency graph given μ from N. It can be seen from the figure that the maximum permeability value is reached somewhat earlier than saturation. With an unlimited increase H permeably asymptotically approaches unity. This follows from the fact that / in the expression μ = 1 - J/ H cannot exceed 1.
|
|
|
Figure - 3.41 |
Quantities AT r (or J r ), N with and μ are the main characteristics of a ferromagnet. If the coercive force H with has a great the value of a ferromagnet is called tough. It has a wide hysteresis loop. ferromagnet with small H with (and, accordingly, a narrow hysteresis loop) is called soft. Depending on the purpose, ferromagnets with one or another characteristic are taken. So, for permanent magnets, he used hard ferromagnets, and soft ones for transformer cores. The presence of the Curie point in ferromagnets can be understood, given that the atoms participate in thermal motion: as long as the temperature is low, the atoms retain the parallel orientation of their magnetic moments within the domains. But as the temperature increases, the thermal motion also increases. When a substance reaches a certain temperature for a given substance, thermal motion destroys this orientation - the domain disappears. Further, the ferromagnet behaves like a paramagnet.
The foundations of the theory of ferromagnetism were created by Ya. I. Frenkel and W. Heisenberg in 1928. In our time, magnets and their magnetic properties are widely used in science and technology.
If an object is placed in a magnetic field, then its “behavior” and the type of internal structural changes will depend on the material from which the object is made. All known substances can be divided into five main groups: paramagnets, ferromagnets and antiferromagnets, ferrimagnets and diamagnets. In accordance with this classification, the magnetic properties of a substance are distinguished. To understand what is hidden behind these terms, we will consider each group in more detail.
Substances that exhibit the properties of paramagnetism are characterized by magnetic permeability with a positive sign, and regardless of the value of the strength of the external magnetic field in which the object is located. The most famous representatives of this group are gaseous oxygen, metals of the alkaline earth and alkali groups, as well as ferrous salts.
A high magnetic susceptibility of a positive sign (reaches 1 million) is inherent in ferromagnets. Depending on the intensity of the external field and temperature, the susceptibility varies over a wide range. It is important to note that since the moments of elementary particles of different sublattices in the structure are equal, the total value of the moment is zero.
Both in name and in some properties, ferrimagnetic substances are close to them. They are united by a high dependence of the susceptibility on heating and the value of the field strength, but there are also differences. atoms placed in the sublattices are not equal to each other, therefore, unlike the previous group, the total moment is nonzero. The substance is inherent in spontaneous magnetization. The connection of sublattices is antiparallel. The best known are ferrites. The magnetic properties of substances of this group are high, so they are often used in technology.
Of particular interest is the group of antiferromagnets. When such substances are cooled below a certain temperature limit, the atoms and their ions located in the structure of the crystal lattice naturally change their magnetic moments, acquiring an antiparallel orientation. A completely different process takes place when a substance is heated - it registers magnetic properties characteristic of a group of paramagnets. Examples are carbonates, oxides, etc.
Numerous experiments indicate that all substances placed in a magnetic field are magnetized and create their own magnetic field, the action of which is added to the action of an external magnetic field:
where is the magnetic induction of the field in the substance; - magnetic induction of the field in vacuum, - magnetic induction of the field due to the magnetization of matter.
In this case, the substance can either strengthen or weaken the magnetic field. The influence of a substance on an external magnetic field is characterized by a value called the magnetic permeability of the substance
Magnetic permeability is a physical scalar value showing how many times the magnetic field induction in a given substance differs from the magnetic field induction in vacuum.
Substances that weaken an external magnetic field are called diamagnets(bismuth, nitrogen, helium, carbon dioxide, water, silver, gold, zinc, cadmium, etc.).
Substances that enhance the external magnetic field - paramagnets(aluminum, oxygen, platinum, copper, calcium, chromium, manganese, cobalt salts, etc.).
For diamagnets >1. But in both cases, the difference from 1 is small (several ten-thousandths or hundred-thousandths of a unit). So, for example, bismuth = 0.9998 = 1.000.
Some substances (iron, cobalt, nickel, gadolinium and various alloys) cause a very large increase in the external field. They are called ferromagnets. For them = 10 3 -10 5 .
For the first time, Ampère gave an explanation of the reasons due to which bodies have magnetic properties. According to his hypothesis, elementary electric currents circulate inside molecules and atoms, which determine the magnetic properties of any substance.
It has now been established that all atoms and elementary particles really have magnetic properties. The magnetic properties of atoms are mainly determined by their constituent electrons.
According to the semiclassical model of the atom proposed by E. Rutherford and N. Bohr, electrons in atoms move around the nucleus in closed orbits (in the first approximation, we can assume that they are circular). The movement of an electron can be represented as an elementary circular current, where e is the charge of the electron, v is the frequency of rotation of the electron in orbit. This current forms a magnetic field, which is characterized by a magnetic moment, its modulus is determined by the formula , where S is the area of the orbit.
The magnetic moment of an electron due to its motion around the nucleus is called orbital magnetic moment. The orbital magnetic moment is a vector quantity and direction is determined by the right hand screw rule. If the electron moves clockwise (Fig. 1), then the currents are directed counterclockwise (in the direction of movement positive charge), and the vector is perpendicular to the plane of the orbit.

Since the orbits of different electrons in an atom do not coincide, their magnetic moments are directed at different angles to each other. The resulting orbital magnetic moment of a multi-electron atom is equal to the vector sum of the orbital magnetic moments of individual electrons.
Atoms with partially filled electron shells have an uncompensated orbital magnetic moment. In atoms with filled electron shells, it is equal to 0.
In addition to the orbital magnetic moment, the electron also has own (spin) magnetic moment, which was first established by O. Stern and V. Gerlach in 1922. The existence of a magnetic field in an electron was explained by its rotation around its own axis, although one should not literally liken an electron to a rotating charged ball (top).
It has been reliably established that the magnetic field of an electron is the same integral property as its mass and charge. An electron, in a very rough approximation, can be represented as a very small ball surrounded by electric and magnetic fields (Fig. 2). The magnetic fields of all electrons are the same, as are their masses and charges. The spin magnetic moment is a vector directed along the axis of rotation. It can only orient itself in two ways: either along... or against... If there is an external magnetic field in the place where the electron is located, then either along the field or against the field. As shown in quantum physics, only two electrons can be in the same energy state, the spin magnetic moments of which are opposite (Pauli principle).

In multielectron atoms, the spin magnetic moments of individual electrons, as well as the orbital moments, add up as vectors. In this case, the resulting spin magnetic moment of the atom for atoms with filled electron shells is 0.
The total magnetic moment of an atom (molecule) is equal to the vector sum of the magnetic moments (orbital and spin) of the electrons entering the atom (molecule):
Diamagnets consist of atoms that, in the absence of an external magnetic field, do not have their own magnetic moments, since all spin and all orbital magnetic moments are compensated for them.
An external magnetic field does not act on the entire atom of a diamagnet, but acts on individual electrons of the atom, whose magnetic moments are nonzero. Let in this moment the electron velocity makes a certain angle (Fig. 3) with the magnetic induction of the external field.

Due to the component, the Lorentz force (directed towards us in Fig. 3) will act on the electron, which will cause an additional (except for other movements in which the electron participates in the absence of a field) movement in a circle. But this movement is an additional circular current, which will create a magnetic field, characterized by a magnetic moment (induced), directed according to the rule of the right screw towards. As a result, diamagnets weaken the external magnetic field.
Paramagnets are made up of atoms that have a net magnetic moment of the atom. In the absence of an external field, these moments are randomly oriented and the substance as a whole does not create a magnetic field around itself. When paramagnets are placed in a magnetic field, predominant orientation of the vectors along the field (this is prevented by the thermal motion of particles). Thus, the paramagnet is magnetized, creating its own magnetic field, coinciding in direction with the external field and amplifying it. This effect is called paramagnetic. When the external magnetic field is weakened to zero, the orientation of the magnetic moments due to thermal motion is broken and the paramagnet is demagnetized. In paramagnets, a diamagnetic effect is also observed, but it is much weaker than the paramagnetic one.
MAGNETIC PROPERTIES AND STRUCTURE OF SUBSTANCES
Magnetochemistry is a branch of chemistry that studies the magnetic properties of substances, as well as their relationship with the structure of molecules. Its formation as a science can be attributed to the beginning of the 20th century, when the basic laws of magnetism were discovered.
MAGNETIC PROPERTIES OF SUBSTANCES
Magnetism - fundamental property matter. Since ancient times, the property of permanent magnets to attract iron objects has been known. The development of electromagnetism made it possible to create electromagnets stronger than the constants existing in nature. In general, various devices and devices based on the use of electromagnetic phenomena are so widespread that now it is impossible to imagine life without them.
However, not only permanent magnets interact with a magnetic field, but also all other substances. The magnetic field, interacting with matter, changes its magnitude compared to vacuum (here and below, all formulas are written in the SI system):
where µ 0 is the magnetic constant equal to 4p 10 -7 Gn/m, µ is the magnetic permeability of the substance, B is the magnetic induction (in T), H is the magnetic field strength (in A/m). For most substances, m is very close to unity, so in magnetochemistry, where the main object is a molecule, it is more convenient to use the value c, which is called magnetic susceptibility. It can be attributed to a unit of volume, mass or amount of a substance, then it is called, respectively, volumetric (dimensionless) c.v., specific cd(in cm3/g) or molar cm(in cm3/mol) magnetic susceptibility.
Substances can be divided into two categories: those that weaken the magnetic field (c< 0), называются диамагнетиками, те, которые усиливают (c >0), are paramagnets. It can be imagined that in an inhomogeneous magnetic field, a force acts on a diamagnet, pushing it out of the field, and on a paramagnet, on the contrary, it acts on it. This is the basis of the methods discussed below for measuring the magnetic properties of substances. Diamagnets (and this is the vast majority of organic and high-molecular compounds) and mainly paramagnets are the objects of study of magnetochemistry.
Diamagnetism is the most important property of matter, due to the fact that under the influence of a magnetic field, electrons in filled electron shells (which can be represented as small conductors) begin to precess, and, as you know, any movement of an electric charge causes a magnetic field, which, according to the Lenz rule, will be directed as to reduce the impact from the external field. In this case, the electronic precession can be considered as circular currents. Diamagnetism is characteristic of all substances, except for atomic hydrogen, because all substances have paired electrons and filled electron shells.
Paramagnetism is due to unpaired electrons, which are called so because their own magnetic moment (spin) is not balanced by anything (respectively, the spins of paired electrons are directed in opposite directions and compensate each other). In a magnetic field, the spins tend to line up in the direction of the field, strengthening it, although this order is disturbed by chaotic thermal motion. Therefore, it is clear that the paramagnetic susceptibility depends on temperature - the lower the temperature, the higher the value of the susceptibility.
This type of magnetic susceptibility is also called orientational paramagnetism, since its cause is the orientation of elementary magnetic moments in an external magnetic field.
The magnetic properties of electrons in an atom can be described in two ways. In the first method, it is considered that the intrinsic (spin) magnetic moment of the electron does not affect the orbital moment (due to the movement of electrons around the nucleus) or vice versa. More precisely, this mutual influence always exists (spin-orbit interaction), but for 3d ions it is small, and the magnetic properties can be described with sufficient accuracy by two quantum numbers L (orbital) and S (spin). For heavier atoms, such an approximation becomes unacceptable and one more quantum number of the total magnetic moment J is introduced, which can take values from | L+S | before | L-S |
Attention should be paid to the smallness of the magnetic interaction energy (for room temperatures and magnetic fields common in the laboratory, the energy of magnetic interactions is three to four orders of magnitude less than the energy of the thermal motion of molecules).
There are quite a few substances that, when the temperature is lowered, first behave like paramagnets, and then, when a certain temperature is reached, their magnetic properties change dramatically. The best-known example is ferromagnets and the substance from which they are named, iron, whose atomic magnetic moments below the Curie temperature align in the same direction, causing spontaneous magnetization. However, macroscopic magnetization does not occur in the absence of a field, since the sample is spontaneously divided into regions about 1 μm in size, called domains, within which the elementary magnetic moments are directed in the same way, but the magnetizations of different domains are randomly oriented and, on average, compensate each other. The forces that cause a ferromagnetic transition can only be explained using the laws of quantum mechanics.
Antiferromagnets are characterized by the fact that the spin magnetic moments at the antiferromagnetic transition temperature (Néel temperature TN) are ordered in such a way that they cancel each other out.
If the compensation of magnetic moments is incomplete, then such substances are called ferrimagnets, for example Fe2O3 and FeCr2O4. The last three classes of compounds are solids and are studied mainly by physicists. Over the past decades, physicists and chemists have created new magnetic materials.
In a molecule containing an unpaired electron, the remaining (paired) electrons weaken the magnetic field, but the contribution of each of them is two to three orders of magnitude smaller. However, if we want to measure the magnetic properties of unpaired electrons very accurately, then we must introduce the so-called diamagnetic corrections, especially for large organic molecules, where they can reach tens of percent. The diamagnetic susceptibilities of the atoms in a molecule add to each other according to the Pascal-Langevin additivity rule. To do this, the diamagnetic susceptibilities of atoms of each type are multiplied by the number of such atoms in the molecule, and then constitutive corrections are introduced for structural features (double and triple bonds, aromatic rings, etc.). Let us turn to the consideration of how the magnetic properties of substances are experimentally studied.
EXPERIMENTAL MEASUREMENT OF THE MAGNETIC SUSCEPTIBILITY
The main experimental methods for determining the magnetic susceptibility were created in the last century. Gouy's method measures the change in the weight of a sample in a magnetic field compared to its absence.
The Faraday method measures the force acting on a sample in a non-uniform magnetic field.
The main difference between the Gouy method and the Faraday method is that in the first case, inhomogeneity is maintained along the (extended) sample, and in the second case, along the magnetic field.
The Quincke method is used only for liquids and solutions. It measures the change in the height of a liquid column in a capillary under the influence of a magnetic field.
In this case, for diamagnetic liquids, the height of the column decreases, for paramagnetic liquids it increases.
The viscometer method measures the time of fluid flow through a small hole with the magnetic field on (tH) and off (t0). The outflow time of paramagnetic fluids in a magnetic field is noticeably shorter than in the absence of a field, and vice versa for diamagnetic fluids.
Magnetic susceptibility can also be measured using an NMR spectrometer. Note that the chemical shift of the NMR signal is generally determined not only by the screening constant, which is a measure of the electron density on the nucleus under study, but also by the magnetic susceptibility of the sample.
The obtained value of the magnetic susceptibility for paramagnets is determined by the number of unpaired electrons (for one unpaired electron)
Magnetochemical studies make it possible to establish the electronic configuration of transition metal compounds, which form the basis of the chemistry of coordination (complex) compounds.
By measuring the magnetic susceptibility, one can easily judge the degree of oxidation and the geometry of the first coordination sphere in the complex.
It is known that most important in practice chemical reactions occur in solutions, they also include complex formation reactions; therefore, in the next section we will consider the magnetic properties of solutions in which transition metal compounds are realized in the form of complexes.
MAGNETIC SUSCEPTIBILITY OF SOLUTIONS
When moving from solid body to the solution, the magnetic susceptibilities of the solvent and all solutes should be taken into account. In this case, the simplest way to take this into account will be the summation of the contributions of all components of the solution according to the additivity rule. The principle of additivity is one of the fundamental principles in the processing of experimental data. Any deviations from it are more often associated with the fact that the principle of additivity itself is fulfilled, and the components of the solution change their properties. Therefore, it is assumed that the magnetic susceptibility of the solution is equal to the sum of the magnetic susceptibilities of the individual components, taking into account the concentration
It can be seen from a study of the magnetic properties of the same substance in different solvents that they can significantly depend on the nature of the solvent. This can be explained by the entry of solvent molecules into the first coordination sphere and the corresponding change in the electronic structure of the complex, the energies of the d-orbitals (D), and other properties of the solvate complex. Thus, magnetochemistry also makes it possible to study solvation, that is, the interaction of a solute with a solvent.
If the magnetic field affects the properties of the solution, and numerous experimental facts (measurements of density, viscosity, electrical conductivity, proton concentration, magnetic susceptibility) indicate that this is the case, then it should be recognized that the interaction energy of the individual components of the solution and an ensemble of water molecules is quite high, then is comparable to or exceeds the energy of the thermal motion of particles in the solution, which averages out any effect on the solution. Recall that the energy of the magnetic interaction of one particle (molecule) is small compared to the energy of thermal motion. Such an interaction is possible if we accept that in water and aqueous solutions, due to the cooperative nature of hydrogen bonds, large ice-like structural ensembles of water molecules are realized, which can be strengthened or destroyed under the influence of dissolved substances. The formation energy of such "ensembles" is apparently comparable to the energy of thermal motion and under magnetic influence, the solution can remember it and acquire new properties, but Brownian motion or an increase in temperature eliminates this "memory" for some time.
By precisely choosing the concentrations of paramagnetic substances in a diamagnetic solvent, it is possible to create a non-magnetic liquid, that is, one in which the average magnetic susceptibility is zero or in which magnetic fields propagate in exactly the same way as in vacuum. This interesting property has not yet found application in technology.
- In contact with 0
- Google Plus 0
- OK 0
- Facebook 0