Alkanes:
Alkanes are saturated hydrocarbons, in the molecules of which all atoms are connected by single bonds. Formula -
Physical Properties:
- Melting and boiling points increase with molecular weight and main carbon chain length
- Under normal conditions, unbranched alkanes from CH 4 to C 4 H 10 are gases; from C 5 H 12 to C 13 H 28 - liquids; after C 14 H 30 - solids.
- The melting and boiling points decrease from less branched to more branched. So, for example, at 20 °C, n-pentane is a liquid, and neopentane is a gas.
Chemical properties:
· Halogenation
this is one of the substitution reactions. The least hydrogenated carbon atom is halogenated first (tertiary atom, then secondary, primary atoms are halogenated last). Halogenation of alkanes takes place in stages - no more than one hydrogen atom is replaced in one stage:
- CH 4 + Cl 2 → CH 3 Cl + HCl (chloromethane)
- CH 3 Cl + Cl 2 → CH 2 Cl 2 + HCl (dichloromethane)
- CH 2 Cl 2 + Cl 2 → CHCl 3 + HCl (trichloromethane)
- CHCl 3 + Cl 2 → CCl 4 + HCl (tetrachloromethane).
Under the action of light, the chlorine molecule decomposes into radicals, then they attack the alkane molecules, taking a hydrogen atom from them, as a result of which methyl radicals CH 3 are formed, which collide with chlorine molecules, destroying them and forming new radicals.
· Combustion
The main chemical property of saturated hydrocarbons, which determine their use as a fuel, is the combustion reaction. Example:
CH 4 + 2O 2 → CO 2 + 2H 2 O + Q
In the event of a lack of oxygen, instead of carbon dioxide, carbon monoxide or coal is obtained (depending on the oxygen concentration).
In general, the combustion reaction of alkanes can be written as follows:
FROM n H 2 n+2 +(1,5n+0.5)O 2 \u003d n CO 2 + ( n+1) H 2 O
· Decomposition
Decomposition reactions occur only under the influence of high temperatures. An increase in temperature leads to the breaking of the carbon bond and the formation of free radicals.
Examples:
CH 4 → C + 2H 2 (t > 1000 °C)
C 2 H 6 → 2C + 3H 2
Alkenes:
Alkenes are unsaturated hydrocarbons containing in the molecule, in addition to single bonds, one double carbon-carbon bond. The formula is C n H 2n
The belonging of a hydrocarbon to the class of alkenes is reflected by the generic suffix -ene in its name.
Physical Properties:
- The melting and boiling points of alkenes (simplified) increase with molecular weight and length of the main carbon chain.
- Under normal conditions, alkenes from C 2 H 4 to C 4 H 8 are gases; from C 5 H 10 to C 17 H 34 - liquids, after C 18 H 36 - solids. Alkenes are insoluble in water, but readily soluble in organic solvents.
Chemical properties:
· Dehydration is the process of splitting a water molecule from an organic compound molecule.
· Polymerization- this is a chemical process of combining many initial molecules of a low molecular weight substance into large polymer molecules.
Polymer is a high molecular weight compound, the molecules of which consist of many identical structural units.
Alkadienes:
Alkadienes are unsaturated hydrocarbons containing in the molecule, in addition to single bonds, two double carbon-carbon bonds. The formula is
. Dienes are structural isomers of alkynes.Physical Properties:
Butadiene is a gas (tboiling −4.5 °C), isoprene is a liquid boiling at 34 °C, dimethylbutadiene is a liquid boiling at 70 °C. Isoprene and other diene hydrocarbons are able to polymerize into rubber. Natural rubber in its purified state is a polymer with the general formula (C5H8)n and is obtained from the latex of certain tropical plants.
Rubber is highly soluble in benzene, gasoline, carbon disulfide. At low temperature it becomes brittle, when heated it becomes sticky. To improve the mechanical and chemical properties of rubber, it is converted into rubber by vulcanization. To obtain rubber products, they are first molded from a mixture of rubber with sulfur, as well as with fillers: soot, chalk, clay and some organic compounds that serve to accelerate vulcanization. Then the products are heated - hot vulcanization. During vulcanization, sulfur chemically bonds with rubber. In addition, in vulcanized rubber, sulfur is contained in a free state in the form of tiny particles.
Diene hydrocarbons are easily polymerized. The polymerization reaction of diene hydrocarbons underlies the synthesis of rubber. Enter into addition reactions (hydrogenation, halogenation, hydrohalogenation):
H 2 C \u003d CH-CH \u003d CH 2 + H 2 -> H 3 C-CH \u003d CH-CH 3
Alkynes:
Alkynes are unsaturated hydrocarbons whose molecules contain, in addition to single bonds, one triple carbon-carbon bond. Formula-C n H 2n-2
Physical Properties:
Alkynes are similar in physical properties to the corresponding alkenes. Lower (up to C 4) - gases without color and odor, having higher boiling points than their counterparts in alkenes.
Alkynes are poorly soluble in water, better in organic solvents.
Chemical properties:
halogenation reactions
Alkynes are capable of adding one or two halogen molecules to form the corresponding halogen derivatives:
Hydration
In the presence of mercury salts, alkynes add water to form acetaldehyde (for acetylene) or ketone (for other alkynes)
The structure of alkanes
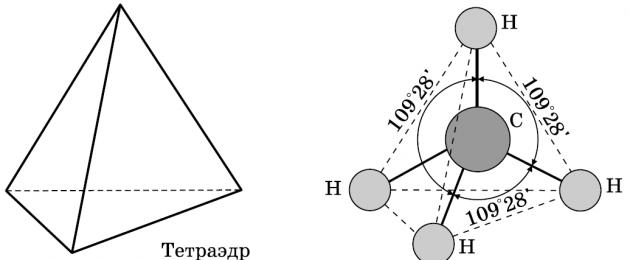
Alkanes are hydrocarbons in whose molecules the atoms are linked by single bonds and which correspond to the general formula C n H 2n+2. In alkane molecules, all carbon atoms are in the state sp 3 hybridization.
This means that all four hybrid orbitals of the carbon atom are the same in shape, energy and directed to the corners of an equilateral triangular pyramid - tetrahedron. The angles between the orbitals are 109° 28'. Practically free rotation is possible around a single carbon-carbon bond, and alkane molecules can take on a wide variety of shapes with angles at carbon atoms close to tetrahedral (109° 28'), for example, in the n-pentane molecule.

It is especially worth recalling the bonds in the molecules of alkanes. All bonds in the molecules of saturated hydrocarbons are single. The overlap occurs along the axis connecting the nuclei of atoms, i.e. this σ-bonds. Carbon-carbon bonds are non-polar and poorly polarizable. The length of the C-C bond in alkanes is 0.154 nm (1.54 10 10 m). C-H bonds are somewhat shorter. The electron density is slightly shifted towards the more electronegative carbon atom, i.e. the C-H bond is weakly polar.
Homologous series of methane
homologues Substances that are similar in structure and properties but differ in one or more CH groups 2 .


Limit hydrocarbons constitute the homologous series of methane.
Isomerism and nomenclature of alkanes
Alkanes are characterized by the so-called structural isomerism. Structural isomers differ from each other in the structure of the carbon skeleton. The simplest alkane, which is characterized by structural isomers, is butane.

Let us consider in more detail for alkanes the basics of nomenclature IUPAC.
1. Main circuit selection. The formation of the name of a hydrocarbon begins with the definition of the main chain - the longest chain of carbon atoms in the molecule, which is, as it were, its basis.
2. Atom numbering of the main chain. The atoms of the main chain are assigned numbers. The numbering of atoms of the main chain starts from the end closest to the substituent (structures A, B). If the substituents are at an equal distance from the end of the chain, then the numbering starts from the end at which there are more of them (structure B). If different substituents are at an equal distance from the ends of the chain, then the numbering starts from the end to which the older one is closer (structure D). The seniority of hydrocarbon substituents is determined by the order in which the letter with which their name begins follows in the alphabet: methyl (-CH 3), then propyl (-CH 2 -CH 2 -CH 3), ethyl (-CH 2 -CH 3 ) etc.
Note that the name of the substituent is formed by replacing the suffix -an with the suffix -yl in the name of the corresponding alkane.
3. Name formation. Numbers are indicated at the beginning of the name - the numbers of carbon atoms at which the substituents are located. If there are several substituents at a given atom, then the corresponding number in the name is repeated twice separated by a comma (2,2-). After the number, a hyphen indicates the number of substituents (di - two, three - three, tetra - four, penta - five) and the name of the substituent (methyl, ethyl, propyl). Then without spaces and hyphens - the name of the main chain. The main chain is called as a hydrocarbon - a member of the homologous series of methane (methane, ethane, propane, etc.).




The names of the substances whose structural formulas are given above are as follows:
Structure A: 2-methylpropane;
Structure B: 3-ethylhexane;
Structure B: 2,2,4-trimethylpentane;
Structure D: 2-methyl 4-ethylhexane.
The absence of saturated hydrocarbons in molecules polar bonds leads to them poorly soluble in water, do not interact with charged particles (ions). The most typical reactions for alkanes are reactions involving free radicals.
Physical properties of alkanes
The first four representatives of the homologous series of methane - gases. The simplest of them is methane - a colorless, tasteless and odorless gas (the smell of "gas", having felt which you need to call 04, is determined by the smell of mercaptans - sulfur-containing compounds specially added to methane used in household and industrial gas appliances so that people those near them could smell the leak).
Composition hydrocarbons from FROM 5 H 12 before FROM 15 H 32 - liquids; heavier hydrocarbons are solids. The boiling and melting points of alkanes gradually increase with increasing carbon chain length. All hydrocarbons are poorly soluble in water; liquid hydrocarbons are common organic solvents.


Chemical properties of alkanes
substitution reactions.
The most characteristic reactions for alkanes are the reactions free radical substitution, during which a hydrogen atom is replaced by a halogen atom or some group.
Let us present the equations of the characteristic halogenation reactions:
In the case of an excess of halogen, chlorination can go further, up to the complete replacement of all hydrogen atoms by chlorine:

The resulting substances are widely used as solvents and starting materials in organic synthesis.
Dehydrogenation reaction(hydrogen splitting).
During the passage of alkanes over the catalyst (Pt, Ni, Al 2 O 3, Cr 2 O 3) at a high temperature (400-600 ° C), a hydrogen molecule is split off and the formation alkene:
Reactions accompanied by the destruction of the carbon chain. All saturated hydrocarbons are burning with the formation of carbon dioxide and water. Gaseous hydrocarbons mixed with air in certain proportions can explode.
1. Combustion of saturated hydrocarbons is a free radical exothermic reaction, which is very important when using alkanes as a fuel:
In general, the combustion reaction of alkanes can be written as follows:
2. Thermal breakdown of hydrocarbons.
The process runs on free radical mechanism. An increase in temperature leads to a homolytic rupture of the carbon-carbon bond and the formation of free radicals.
These radicals interact with each other, exchanging a hydrogen atom, with the formation of a molecule alkane and alkene molecules:
Thermal cleavage reactions are at the heart of the industrial process - hydrocarbon cracking. This process is the most important stage of oil refining.
3. Pyrolysis. When methane is heated to a temperature of 1000 °C, methane pyrolysis- decomposition into simple substances:
When heated to a temperature of 1500 ° C, the formation of acetylene:
4. Isomerization. When linear hydrocarbons are heated with an isomerization catalyst (aluminum chloride), substances are formed with branched carbon skeleton:

5. Aromatization. Alkanes with six or more carbon atoms in the chain in the presence of a catalyst are cyclized to form benzene and its derivatives:

Alkanes enter into reactions that proceed according to the free radical mechanism, since all carbon atoms in alkane molecules are in a state of sp 3 hybridization. The molecules of these substances are built using covalent non-polar C-C (carbon - carbon) bonds and weakly polar C-H (carbon - hydrogen) bonds. They do not have areas with high and low electron density, easily polarizable bonds, i.e., such bonds, the electron density in which can be shifted under the influence of external factors (electrostatic fields of ions). Consequently, alkanes will not react with charged particles, since bonds in alkane molecules are not broken by a heterolytic mechanism.
Limit hydrocarbons are such compounds that are molecules consisting of carbon atoms in the sp 3 hybridization state. They are linked exclusively by covalent sigma bonds. The name "saturated" or "saturated" hydrocarbons comes from the fact that these compounds do not have the ability to attach any atoms. They are ultimate, fully saturated. The exception is cycloalkanes.
What are alkanes?
Alkanes are saturated hydrocarbons, and their carbon chain is open and consists of carbon atoms linked together by single bonds. It does not contain other (that is, double, like in alkenes, or triple, like in alkyls) bonds. Alkanes are also called paraffins. They received this name, since the well-known paraffins are a mixture of mainly these saturated hydrocarbons C 18 -C 35 with a special inertness.
General information about alkanes and their radicals
Their formula: C n P 2 n +2, here n is greater than or equal to 1. The molar mass is calculated by the formula: M = 14n + 2. A characteristic feature: the endings in their names are “-an”. The remains of their molecules, which are formed as a result of the replacement of hydrogen atoms with other atoms, are called aliphatic radicals, or alkyls. They are denoted by the letter R. The general formula of monovalent aliphatic radicals: C n P 2 n +1, here n is greater than or equal to 1. The molar mass of aliphatic radicals is calculated by the formula: M = 14n + 1. A characteristic feature of aliphatic radicals: endings in the names “- silt". Alkane molecules have their own structural features:
- the C-C bond is characterized by a length of 0.154 nm;
- the C-H bond is characterized by a length of 0.109 nm;
- the bond angle (the angle between carbon-carbon bonds) is 109 degrees and 28 minutes.

Alkanes begin the homologous series: methane, ethane, propane, butane, and so on.
Physical properties of alkanes
Alkanes are substances that are colorless and insoluble in water. The temperature at which alkanes begin to melt and the temperature at which they boil increase in accordance with the increase in molecular weight and length of the hydrocarbon chain. From less branched to more branched alkanes, the boiling and melting points decrease. Gaseous alkanes are capable of burning with a pale blue or colorless flame, and quite a lot of heat is released. CH 4 -C 4 H 10 are gases that also lack odor. C 5 H 12 -C 15 H 32 are liquids that have a specific odor. C 15 H 32 and so on are solids that are also odorless.
Chemical properties of alkanes
These compounds are chemically inactive, which can be explained by the strength of hard-to-break sigma bonds - C-C and C-H. It is also worth considering that C-C bonds are non-polar, and C-H are slightly polar. These are low-polarizable types of bonds related to the sigma type and, accordingly, they will most likely break by the homolytic mechanism, as a result of which radicals will be formed. Thus, the chemical properties of alkanes are mainly limited to radical substitution reactions.

Nitration reactions
Alkanes interact only with nitric acid at a concentration of 10% or with tetravalent nitric oxide in a gaseous medium at a temperature of 140°C. The nitration reaction of alkanes is called the Konovalov reaction. As a result, nitro compounds and water are formed: CH 4 + nitric acid (diluted) \u003d CH 3 - NO 2 (nitromethane) + water.
Combustion reactions
Limit hydrocarbons are very often used as fuel, which is justified by their ability to burn: C n P 2n + 2 + ((3n + 1) / 2) O 2 \u003d (n + 1) H 2 O + n CO 2.
Oxidation reactions
The chemical properties of alkanes also include their ability to oxidize. Depending on what conditions accompany the reaction and how they are changed, it is possible to obtain different end products from the same substance. Mild oxidation of methane with oxygen in the presence of a catalyst that accelerates the reaction and a temperature of about 200 ° C can result in the following substances:
1) 2CH 4 (oxygen oxidation) = 2CH 3 OH (alcohol - methanol).
2) CH 4 (oxidation with oxygen) \u003d CH 2 O (aldehyde - methanal or formaldehyde) + H 2 O.
3) 2CH 4 (oxidation with oxygen) \u003d 2HCOOH (carboxylic acid - methane or formic) + 2H 2 O.

Also, the oxidation of alkanes can be carried out in a gaseous or liquid medium with air. Such reactions lead to the formation of higher fatty alcohols and corresponding acids.
Relation to heat
At temperatures not exceeding + 150-250 ° C, necessarily in the presence of a catalyst, a structural rearrangement of organic substances occurs, which consists in changing the order of connection of atoms. This process is called isomerization, and the substances obtained as a result of the reaction are called isomers. Thus, from normal butane, its isomer, isobutane, is obtained. At temperatures of 300-600 ° C and the presence of a catalyst, C-H bonds are broken with the formation of hydrogen molecules (dehydrogenation reactions), hydrogen molecules with the carbon chain closed in a cycle (alkanes cyclization or aromatization reactions):
1) 2CH 4 \u003d C 2 H 4 (ethene) + 2H 2.
2) 2CH 4 \u003d C 2 H 2 (ethyne) + 3H 2.
3) C 7 H 16 (normal heptane) \u003d C 6 H 5 - CH 3 (toluene) + 4H 2.

Halogenation reactions
Such reactions consist in the introduction of halogens (their atoms) into the molecule of organic matter, as a result of which a C-halogen bond is formed. When alkanes react with halogens, halogen derivatives are formed. This reaction has specific features. It proceeds according to a radical mechanism, and in order to initiate it, it is necessary to influence the mixture of halogens and alkanes with ultraviolet radiation or simply heat it. The properties of alkanes allow the halogenation reaction to proceed until complete substitution with halogen atoms is achieved. That is, the chlorination of methane will not end with one stage and the production of methyl chloride. The reaction will go further, all possible substitution products will be formed, starting with chloromethane and ending with carbon tetrachloride. The action of chlorine under these conditions on other alkanes will lead to the formation of various products obtained as a result of the substitution of hydrogen at various carbon atoms. The temperature at which the reaction takes place will determine the ratio of the final products and the rate of their formation. The longer the hydrocarbon chain of an alkane, the easier this reaction will go. In halogenation, the least hydrogenated (tertiary) carbon atom will be replaced first. The primary will react after all the others. The halogenation reaction will proceed in stages. At the first stage, only one hydrogen atom is replaced. Alkanes do not react with halogen solutions (chlorine and bromine water).

Sulfochlorination reactions
The chemical properties of alkanes are also supplemented by the sulfochlorination reaction (it is called the Reed reaction). When exposed to ultraviolet radiation, alkanes are able to react with a mixture of chlorine and sulfur dioxide. As a result, hydrogen chloride is formed, as well as an alkyl radical, which attaches sulfur dioxide to itself. The result is a complex compound that becomes stable due to the capture of a chlorine atom and the destruction of its next molecule: R-H + SO 2 + Cl 2 + ultraviolet radiation = R-SO 2 Cl + HCl. The sulfonyl chlorides formed as a result of the reaction are widely used in the production of surfactants.
Limit hydrocarbons, or paraffins, are such biocompounds, in the molecules of which the carbon atoms are connected by a simple (single) bond, and all other valence units are saturated with hydrogen atoms.
Alkanes: physical properties
The removal of hydrogen from an alkane molecule, or dehydrogenation, in the presence of catalysts and when heated (up to 460 ° C), makes it possible to obtain the necessary alkenes. Methods have been developed for the oxidation of alkanes at low temperatures in the presence of catalysts (magnesium salts). This makes it possible to directly influence the course of the reaction and obtain the necessary oxidation products in the course of chemical synthesis. For example, the oxidation of higher alkanes produces a variety of higher alcohols or higher fatty acids.
The splitting of alkanes also occurs under other conditions (combustion, cracking). Saturated hydrocarbons burn with a blue flame, releasing enormous amounts of heat. These properties make it possible to use them as a high-calorie fuel both in everyday life and in industry.
Page 1
Chemical properties of alkanes.
All bonds in alkanes are of low polarity; therefore, they are characterized by radical reactions. The absence of pi bonds makes addition reactions impossible. Alkanes are characterized by substitution, elimination, and combustion reactions.
|
Type and name of the reaction |
Example |
|
1. Substitution reactions | |
|
A) with halogens(from chlorineCl 2 – in the light, Br 2 - when heated) the reaction obeys Markovnik's rule (Markovnikov's rules ) - primarily a halogenreplaces hydrogen atleast hydrogenated a carbon atom. Reaction takes place in stages - in one stage replaced no more than one hydrogen atom. Iodine reacts most difficultly, and moreover, the reaction does not go to the end, since, for example, when methane reacts with iodine, hydrogen iodide is formed, which reacts with methyl iodide to form methane and iodine (reversible reaction): |
CH 4 + Cl 2 → CH 3 Cl + HCl (chloromethane) CH 3 Cl + Cl 2 → CH 2 Cl 2 + HCl (dichloromethane) CH 2 Cl 2 + Cl 2 → CHCl 3 + HCl (trichloromethane) CHCl 3 + Cl 2 → CCl 4 + HCl (tetrachloromethane). |
B) Nitration (Konovalov's reaction)Alkanes react with 10% nitric acid solution or nitric oxide N 2 O 4 in the gas phase at a temperature of 140 ° and low pressure with the formation of nitro derivatives. The reaction also obeys Markovnikov's rule. ABOUT one of the hydrogen atoms is replaced by the remainder of NO 2 (nitro group) and water is released | |
|
2. Elimination reactions | |
|
A) dehydrogenation- removal of hydrogen. Reaction conditions catalyst-platinum and temperature. |
CH 3 - CH 3 → CH 2 \u003d CH 2 + H 2
|
|
B) cracking the process of thermal decomposition of hydrocarbons, which is based on the reactions of splitting the carbon chain of large molecules with the formation of compounds with a shorter chain. At a temperature of 450–700 o C alkanes break down by breaking bonds S–S (stronger ties S–N are preserved at this temperature) and alkanes and alkenes with a smaller number of carbon atoms are formed |
C 6 H 14 C 2 H 6 + C 4 H 8 |
|
C) complete thermal decomposition |
CH 4 C + 2H 2 |
|
3. Oxidation reactions | |
|
A) combustion reaction When ignited (t = 600 o C), alkanes react with oxygen, while they are oxidized to carbon dioxide and water. |
С n Н 2n+2 + O 2 ––> CO 2 + H 2 O + Q CH 4 + 2O 2 ––> CO 2 + 2H 2 O + Q |
|
B) Catalytic oxidation-at a relatively low temperature and with the use of catalysts, it is accompanied by the breaking of only a part of the C–C bonds, approximately in the middle of the molecule and C–H, and is used to obtain valuable products: carboxylic acids, ketones, aldehydes, alcohols. |
For example, with incomplete oxidation of butane (breaking the C 2 -C 3 bond), acetic acid is obtained |
|
4. Isomerization reactions not typical for all alkanes. Attention is drawn to the possibility of converting some isomers into others, the presence of catalysts. |
C 4 H 10 C 4 H 10 |
|
5.. Alkanes with 6 or more carbon backbones also reactdehydrocyclization, but always form a 6-membered cycle (cyclohexane and its derivatives). Under the reaction conditions, this cycle undergoes further dehydrogenation and turns into an energetically more stable benzene cycle of an aromatic hydrocarbon (arene). |
 |
Methods for obtaining alkanes.
Alkanes are obtained in large quantities from natural gas and oil.
From simple substances in an electric discharge:
Hydrolysis of aluminum carbide
Heating monohaloalkanes with sodium metal (Wurtz reaction)
If different haloalkanes, then the result will be a mixture of three products:
Decarboxylation. Fusion of sodium acetate with alkali. The alkane obtained in this way will have one less carbon atom.
Hydrolysis of the Grignard reagent:
Alkanes of a symmetrical structure can be obtained by electrolysis of salts of carboxylic acids. (Kolb reaction)
Page 1
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0