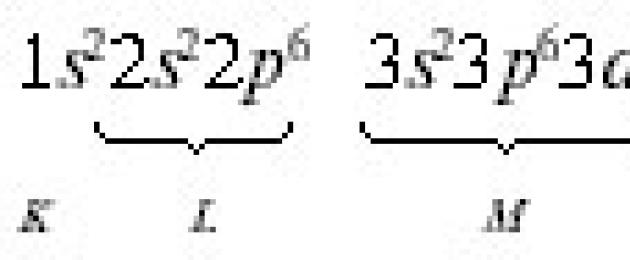The distribution of electrons in an atom is carried out in accordance with 3 provisions of quantum mechanics: the Pauli principle; the principle of minimum energy; Hund's rule.
According to the Pauli principle An atom cannot have two electrons with the same values of all four quantum numbers. The Pauli principle determines the maximum number of electrons in one orbital, level and sublevel. Since AO is characterized by three quantum numbers n, l, ml, the electrons of a given orbital can differ only in their spin quantum number ms. But ms can only have two values +½ and -½.
Consequently, one orbital can contain no more than two electrons with opposite spins. The maximum number of electrons at an energy level is defined as 2 n 2 , and at the sublevel - like 2 (2 l+1). The maximum number of electrons located at different levels and sublevels is given in table. 2.1.
Maximum number of electrons at quantum levels and sublevels
| Energy level | Energy sublevel | Possible values of the magnetic quantum number ml | Number of joint-stock companies in | Maximum number of electrons per | ||
| sublevel | level | sublevel | level | |||
| K (n= 1) | s (l= 0) | |||||
| L (n= 2) | s (l= 0) p (l= 1) | -1, 0, 1 | ||||
| M (n= 3) | s (l= 0) p (l= 1) d (l= 2) | -1, 0, 1 -2, -1, 0, 1, 2 | ||||
| N (n= 4) | s (l= 0) p (l= 1) d (l= 2) f (l= 3) | -1, 0, 1 -2, -1, 0, 1, 2 -3, -2, -1, 0, 1, 2, 3 |
The sequence of filling orbitals with electrons is carried out in accordance with principle of minimum energy, Whereby electrons fill the orbitals in order of increasing energy level of the orbitals. The order of orbitals in energy is determined Klechkovsky's rule : the increase in energy, and accordingly, the filling of orbitals occurs in increasing order of the sum (n + l), and with an equal sum (n + l) - in increasing order of n.
The order of electron distribution among energy levels and sublevels in the shell of an atom it's called electronic configuration. When writing an electronic configuration, the level number (principal quantum number) is denoted by the numbers 1, 2, 3, 4..., the sublevel (orbital quantum number) - by letters s, p, d, f. The number of electrons in a sublevel is indicated by a number, which is written at the top of the sublevel symbol. For example, the electronic configuration of a sulfur atom is 16 S 1 s 2 2s 2 2p 6 3s 2 3p 4, and vanadium 23 V 1 s 2 2s 2 2p 6 3s 2 3p 6 3d°/i> 3 4 s 2 .
The chemical properties of atoms are determined mainly by the structure of the outer energy levels, which are called valence. Fully completed energy levels in chemical interaction do not participate. Therefore, for the sake of brevity in recording the electronic configuration of an atom, they are often denoted by the symbol of the preceding noble gas. So, for sulfur: 3 s 2 3p 4 ; for vanadium: 3 d 3 4s 2. At the same time, the abbreviated notation clearly highlights the valence electrons that determine Chemical properties atoms of the element.
Depending on which sublevel in the atom is filled last, all chemical elements are divided into 4 electronic families: s-, p-, d-, f- elements. Elements whose atoms are the last to fill the s-sublevel of the outer level are called s-elements. U s- valence elements are s-electrons of the outer energy level.
U p-elements, the p-sublevel of the outer level is filled last. Their valence electrons are located on p- And s- sublayers of the outer layer. U d-elements, the d-sublevel of the pre-external level is filled last and valence are s- electrons of the outer and d- electrons of the pre-external energy levels. U f-elements, the last to be filled is the f-sublevel of the third outer energy level.
The electronic configuration of an atom can also be depicted in the form of diagrams of the arrangement of electrons in quantum cells, which are a graphical representation of the atomic orbital. Each quantum cell can contain no more than two electrons with opposite spins. The order of electron placement within one sublevel is determined by Hund's rule: Within a sublevel, electrons are placed so that their total spin is maximum. In other words, the orbitals of a given sublevel are filled first by one electron with the same spins, and then by a second electron with opposite spins.
Total spin R- electrons of the third energy level of the sulfur atom S ms= ½ - ½ + ½ + ½ = 1; d-electrons of the vanadium atom -
S ms= ½ + ½ + ½ = 3 / 2.
Often, not the entire electronic formula is graphically depicted, but only those sublevels on which the valence electrons are located, for example,
16 S…3 s 2 3p 4 ; 23 V…3 d 3 4s 2 .
When graphically depicting the electronic configuration of an atom in an excited state, vacant valence orbitals are depicted along with filled ones. For example, in the phosphorus atom at the third energy level there is one s-AO, three R-AO and five d-AO. The electronic configuration of the phosphorus atom in the ground state has the form
15 R... 3 s 2 3p 3 .
The valence of phosphorus, determined by the number of unpaired electrons, is equal to 3. When an atom transitions to an excited state, the electrons of state 3 are paired s and one of the electrons with s-sublevel can go to d-sublevel:
P*… 3 s 2 3p 3 3d 1
In this case, the valency of phosphorus changes from three (PCl 3) in the ground state to five (PCl 5) in the excited state.
COMPOSITION AND ELECTRONIC
ATOMIC STRUCTURE
METHODOLOGICAL INSTRUCTIONS AND CONTROL TASKS
TO THE TRAINING PROGRAM FOR STUDENTS
SPECIALIZED CLASSES
COMMON EDUCATION SCHOOLS
Continuation. See the beginning in № 4, 6/2005
Guidelines
17. Taking into account the described patterns, consider the state and distribution of electrons across energy levels and orbitals for potassium atoms ( Z= 19) and scandium ( Z = 21).
Solution
1) The element preceding potassium in PSCE is argon ( Z= 18) has the following electron distribution:
a) by atomic levels:
b) according to the orbitals of the atom:
Electronic formula of the argon atom:
![]()
Electron graphic formula of the argon atom:
When distributing electrons in the K atom in accordance with the Klechkovsky rule, preference is given to orbital 4 s(sum of quantum numbers n + l equal to: 4 + 0 = 4) compared to orbital 3 d(sum of quantum numbers n + l equal to: 3 + 2 = 5) as the orbital having the minimum value n + l. Consequently, for the potassium atom, the distribution of electrons over orbitals (electron graphic formula) has the form (see paragraph 16 methodological instructions):
Potassium belongs to s-elements with the following electronic formula (configuration) of the atom:
![]()
The electron energy level distribution for the K atom is shown below:

2) The element preceding scandium in PSCE is calcium ( Z= 20) has the following electron distribution:
a) by atomic levels:

b) according to the orbitals of the atom:
Electronic formula of the calcium atom:

From orbitals 3 d (n + l equals: 3 + 2 = 5) and 4 p (n + l equals: 4 + 1 = 5) when distributing electrons in a scandium atom among orbitals, preference should be given to 3 d-orbital as having the minimum value n= 3 for the same sums of quantum numbers ( n + l) equal to five. Therefore, scandium belongs to d-elements, and its atom is characterized by the following distribution of electrons among orbitals:
Electronic formula of the scandium atom:

The electron energy level distribution for the Sc atom is depicted below:

18. Complete the drawing to show the appearance of one s-orbitals and three R-orbitals oriented along the axes.

Table 5
Electron distribution
by quantum levels and sublevels
| Shell | Energy level n |
Energy sublevel l |
Magnetic number m |
Number orbitals |
Limit number electrons |
||||
|---|---|---|---|---|---|---|---|---|---|
| K | 1 | 0(s) | 0 | 1 | 2 | ||||
| L | 2 | 0(s) 1 (p) |
+1, 0, –1 |
|
|
||||
| M | 3 | 0(s) 1 (p) 2(d) |
0
1, 0, –1 |
|
|
||||
| N | 4 | 0(s) 1 (p) 2(d) 3(f) |
0 +1, 0, –1 +2, +1, 0, –1, –2 +3, +2, +1, 0, –1, –2, –3 |
|
|
20. For the sequence of filling the energy levels of atoms, see table. 6.
21. The number of elements in a period of D.I. Mendeleev’s table is determined by the formulas:
a) for odd periods:
Ln = (n + 1) 2 /2,
b) for even periods:
Ln = (n + 2) 2 /2,
Where Ln– number of elements in the period, n– period number.
Define the number of elements in each period of D.I. Mendeleev’s PSHE.
Explain:
a) the resulting numerical pattern from the standpoint of the state of electrons in atoms and their distribution among energy levels;
b) division of groups of elements into main and secondary subgroups;
c) the predetermination of the number of main and secondary subgroups in D.I. Mendeleev’s PSHE from the point of view of the theory of atomic structure.
Check in the future, your conclusions on Appendix 1 (P-21).
22. The strict periodicity of the arrangement of elements in D.I. Mendeleev’s PSHE is fully explained by the sequential filling of the energy levels of atoms (see paragraph 20 above). Strengthening positions periodic law Based on the patterns of changes in the electronic structure of atoms of elements, first predicted by N. Bohr, the discovery of the 72nd element contributed. Chemists searched for the then-undiscovered element among minerals containing rare earth elements, based on the incorrect premise that 15 elements should be classified as lanthanides.
By analogy with transition elements, the number of lanthanides (elements No. 58–71) should be equal to the difference between the maximum numbers of electrons per N And M energy levels
(32 – 18 = 14), i.e. equal to the maximum number of electrons per f-sublevel (see paragraph 19 above). Element with Z= 72 (hafnium Hf) is an analogue of zirconium Zr and has been found in zirconium ores.
23. Next important conclusion from the analysis of table. 6 in paragraph 20 is the conclusion about the periodicity of filling the external energy levels of atoms with electrons, which determines the periodicity of changes in the chemical properties of elements and their compounds.
Table 6
Electronic configurations of atoms
first 20 elements of the periodic table
| Atomic number |
Oboz- meaning |
Layer | K | L | M | N |
| n | 1 | 2 | 3 | 4 | ||
| l | 0 | 0, 1 | 0, 1, 2 | 0, 1, 2, 3 | ||
| Sublevel | 1s | 2s, 2p | 3s, 3p, 3d | 4s, 4p, 4d, 4f | ||
| Number of electrons at a given sublevel | ||||||
| 1 2 |
H He |
1 2 |
||||
| 3 4 5 6 7 8 9 10 |
Li Be B C N O F Ne |
2 2 2 2 2 2 2 2 |
1, 0 2, 0 2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 |
|||
| 11 12 13 14 15 16 17 18 |
Na Mg Al Si P S Cl Ar |
2 2 2 2 2 2 2 2 |
2, 6 2, 6 2, 6 2, 6 2, 6 2, 6 2, 6 2, 6 |
1, 0, 0 2, 0, 0 2, 1, 0 2, 2, 0 2, 3, 0 2, 4, 0 2, 5, 0 2, 6, 0 |
||
| 19 20 |
K Ca |
2 2 |
2, 6 2, 6 |
2, 6, 0 2, 6, 0 |
1, 0, 0, 0 2, 0, 0, 0 |
|
Thus, the second period of D.I. Mendeleev’s table consists of eight elements with the following sublevels:
|
During the transition from lithium to neon, the charge of the atomic nucleus gradually increases from Z= 3 to Z= 10, which means that the forces of attraction of electrons to the nucleus increase, and as a result, the radii of the atoms of these elements decrease. Therefore, the ability of an atom to donate electrons (a typically metallic property), pronounced in the lithium atom, gradually weakens when moving from lithium to fluorine. The latter is a typical non-metal, that is, an element more capable than others of acquiring electrons.
Starting from the element next to neon (Na, Z= 11) the electronic structures of atoms are repeated, and therefore the electronic configurations of their outer electron shells are designated in a similar way ( n– period number):
ns 1 (Li,Na), ns 2 (Be, Mg), ns 2 n.p. 1 (B, Al), ns 2 n.p. 2 (C, Si) etc.
In the fourth period of D.I. Mendeleev’s table, transition elements appear that belong to secondary subgroups.
24. Elements belonging to the same subgroup have a similar arrangement of electrons in the outer electronic levels of the atoms. For example, the halogen atoms (the main subgroup of group VII) all have the electronic configuration ns 2 n.p. 5, and the atoms of elements of a side subgroup of the same group are characterized by an electronic configuration ( n– 1)s 2 (n– 1)p 6 (n– 1)d 5 ns 2 .
What is the essence of the similarities and differences between atoms of elements belonging to different subgroups of the same group of D.I. Mendeleev’s table? In the future, check your conclusions with Appendix 1 (P-24).
25. The numerical value of the valency of an atom, determined by the number of covalent chemical bonds formed by it, reflects the position of the element in D.I. Mendeleev’s PSCE. In many cases, the valency of an element atom in a compound is numerically equal to the group number in D.I. Mendeleev’s PSHE. However, there are exceptions to this rule. For example, the phosphorus atom on the outer (third, M) energy level contains three unpaired electrons (3 R-orbitals) and free valence cells d-orbitals. Consequently, the phosphorus atom is characterized by the so-called excitation electron, associated with the pairing of an electron pair and the transition of one of the resulting unpaired electrons to 3 d-orbital. For the excited state of the phosphorus atom, the formation of five covalent bonds, and for the main one - only three.

For the nitrogen atom, the excited state is atypical, since in this atom at the external energy level the number and state of electrons is the same as in the phosphorus atom, but there are no vacant cells, and only three electrons are missing for the completion and stability of this level.
Why then is the maximum valency of the nitrogen atom in compounds (i.e., the ability to form common electron pairs) not III, but IV?
26. Repeating paragraphs. 16, 17 methodological development, we can explain the order in which electrons fill energy levels in the atoms of elements of the 4th long period PSHE D.I.Mendeleev. The even series of this period begins with elements of the main subgroups - 39 K and 40 Ca, which are typical metals with constant valency, and already with element No. 21 ( Z= 21, Sc) then there are elements of side subgroups called d- elements or transitional. Try to explain the essence of these names and give relevant examples. In the future, check the correctness of your conclusions with Appendix 1 (P-26).
27.
The chemical symbol of hydrogen H in D.I. Mendeleev’s PSHE is also placed in the main subgroup
Group I, and to the main subgroup of Group VII. Why is this acceptable? Check in the future the correctness of your conclusions in Appendix 1 (P-27).
Mendeleev's periodic table of elements.
Periodic table of chemical elements (Mendeleev table) - classification chemical elements, establishing the dependence of various properties of elements on the charge of the atomic nucleus.
Groups
A group, or family, is one of the columns periodic table. Groups, as a rule, are characterized by more significantly expressed periodic trends than periods or blocks.
In accordance with international system The naming groups are assigned numbers from 1 to 18 in the direction from left to right - from alkali metals to noble gases.
Periods
Period is a row of the periodic table. Within a period, elements show certain patterns in all three aspects mentioned above (atomic radius, ionization energy and electronegativity), as well as in electron affinity energy.
Blocks
Because of the importance of the outer electron shell of an atom, different regions of the periodic table are sometimes described as blocks, named according to which shell contains the last electron. The S-block includes the first two groups, that is, alkaline and alkaline earth metals, as well as hydrogen and helium; The p-block consists of the last six groups (13 to 18 according to the IUPAC naming standard, or IIIA to VIIIA according to the American system) and includes, among other elements, all metalloids. D-block is groups from 3 to 12 (IUPAC), they are also from IIIB to IIB in American, which includes all transition metals. The F-block, usually taken out of the table, consists of lanthanides and actinides.
The periodic table of D.I. Mendeleev became the most important milestone in the development of atomic-molecular science. Thanks to her it worked out modern concept about a chemical element, ideas about simple substances and compounds were clarified.
Composition and characteristics of the atomic nucleus.
Atomic nucleus- the central part of the atom, in which the bulk of its mass is concentrated (more than 99.9%). The nucleus is positively charged; the charge of the nucleus is determined by the chemical element to which the atom belongs.
The atomic nucleus consists of nucleons - positively charged protons and neutral neutrons, which are connected to each other through the strong interaction.
The atomic nucleus, considered as a class of particles with a certain number of protons and neutrons, is usually called nuclide.
The number of protons in a nucleus is called its charge number - this number is equal to the atomic number of the element to which the atom belongs in Mendeleev’s table (Periodic Table of Elements). The number of protons in the nucleus determines the structure of the electron shell of a neutral atom and, thus, the chemical properties of the corresponding element. The number of neutrons in a nucleus is called its isotopic number. Nuclei with the same number of protons and different numbers neutrons are called isotopes. Nuclei with the same number of neutrons but different numbers of protons are called isotones.
The total number of nucleons in a nucleus is called its mass number () and is approximately equal to the average mass of an atom shown in the periodic table. Nuclides with the same mass number but different proton-neutron composition are usually called isobars.
Weight
Due to the difference in the number of neutrons, isotopes of an element have different masses, which is an important characteristic of the nucleus. In nuclear physics, the mass of nuclei is usually measured in atomic mass units ( A. eat.), for one a. e.m. take 1/12 of the mass of the nuclide 12 C [sn 2]. It should be noted that the standard mass that is usually given for a nuclide is the mass of a neutral atom. To determine the mass of the nucleus, you need to subtract the sum of the masses of all electrons from the mass of the atom (a more accurate value will be obtained if you also take into account the binding energy of the electrons with the nucleus).
In addition, in nuclear physics the energy equivalent of mass is often used. According to Einstein's relation, each mass value corresponds to total energy:
Where is the speed of light in vacuum.
The relationship between a. e.m. and its energy equivalent in joules:
and since 1 electronvolt = 1.602176·10 −19 J, then the energy equivalent is a. e.m. in MeV is equal to
Radius
Analysis of the decay of heavy nuclei clarified Rutherford's estimate [sn 3] and related the radius of the nucleus to the mass number by a simple relation:
where is a constant.
Since the radius of the core is not purely geometric characteristic and is associated primarily with the radius of action of nuclear forces, the value depends on the process during the analysis of which the value was obtained, the average value of m, thus the radius of the nucleus in meters
Charge
The number of protons in a nucleus directly determines its electric charge, isotopes have the same number of protons, but different numbers of neutrons. .
The charges of atomic nuclei were first determined by Henry Moseley in 1913. The scientist interpreted his experimental observations by the dependence of the X-ray wavelength on a certain constant, varying by one from element to element and equal to one for hydrogen:
![]() , Where
, Where
And - permanent.
Nuclear binding energy.
The binding energy of the nucleus is equal to the minimum energy that must be expended for complete splitting nuclei into individual particles. From the law of conservation of energy it follows that the binding energy is equal to the energy that is released during the formation of a nucleus from individual particles.
The binding energy of any nucleus can be determined by accurately measuring its mass. Currently, physicists have learned to measure the masses of particles - electrons, protons, neutrons, nuclei, etc. - with very high accuracy. These measurements show that mass of any nucleus M I is always less than the sum of the masses of its constituent protons and neutrons:
This energy is released during the formation of a nucleus in the form of γ-quanta radiation.
Nuclear forces.
Nuclear forces are short-acting forces. They appear only at very small distances between nucleons in the nucleus of the order of 10 –15 m. The length (1.5 – 2.2) 10 –15 m is called range of nuclear forces.
Nuclear forces discover charge independence : The attraction between two nucleons is the same regardless of the charge state of the nucleons - proton or neutron. The charge independence of nuclear forces is visible from a comparison of binding energies mirror cores . This is what the kernels are called,in which the same total number nucleons,but the number of protons in one is equal to the number of neutrons in the other.
Nuclear forces have saturation property , which manifests itself in, that a nucleon in a nucleus interacts only with a limited number of neighboring nucleons. That is why it is observed linear dependence binding energies of nuclei from their mass numbers A. Almost complete saturation of nuclear forces is achieved in the α-particle, which is a very stable formation.
Nuclear forces depend on spin orientations interacting nucleons. This is confirmed by the different nature of neutron scattering by ortho- and parahydrogen molecules. In an orthohydrogen molecule, the spins of both protons are parallel to each other, while in a parahydrogen molecule they are antiparallel. Experiments have shown that neutron scattering on parahydrogen is 30 times greater than scattering on orthohydrogen. Nuclear forces are not central.
So, let's list general properties of nuclear forces :
· small radius of action of nuclear forces ( R~ 1 fm);
· large nuclear potential U~50 MeV;
· dependence of nuclear forces on the spins of interacting particles;
· tensor nature of the interaction of nucleons;
· nuclear forces depend on the mutual orientation of the spin and orbital moments of the nucleon (spin-orbital forces);
· nuclear interaction has the property of saturation;
· charge independence of nuclear forces;
· exchange nature of nuclear interaction;
attraction between nucleons over large distances ( r> 1 fm), is replaced by repulsion at small ( r < 0,5 Фм).
6.6. Features of the electronic structure of atoms of chromium, copper and some other elements
If you carefully looked at Appendix 4, you probably noticed that for atoms of some elements the sequence of filling orbitals with electrons is disrupted. Sometimes these violations are called “exceptions,” but this is not so - there are no exceptions to the laws of Nature!
The first element with this disorder is chromium. Let's take a closer look at its electronic structure (Fig. 6.16 A). The chromium atom has 4 s-there are not two sublevels, as one would expect, but only one electron. But at 3 d-sublevel has five electrons, but this sublevel is filled after 4 s-sublevel (see Fig. 6.4). To understand why this happens, let's look at what electron clouds are 3 d-sublevel of this atom.
Each of five 3 d-clouds in this case are formed by one electron. As you already know from § 4 of this chapter, the total electron cloud of such five electrons has a spherical shape, or, as they say, spherically symmetrical. According to the nature of the distribution of electron density in different directions, it is similar to 1 s-EO. The energy of the sublevel whose electrons form such a cloud turns out to be less than in the case of a less symmetrical cloud. In this case, the orbital energy is 3 d-sublevel is equal to energy 4 s-orbitals. When symmetry is broken, for example, when a sixth electron appears, the energy of the orbitals is 3 d-the sublevel again becomes greater than energy 4 s-orbitals. Therefore, the manganese atom again has a second electron at 4 s-AO.
The general cloud of any sublevel, filled with electrons either half or completely, has spherical symmetry. The decrease in energy in these cases is of a general nature and does not depend on whether any sublevel is half or completely filled with electrons. And if so, then we must look for the next violation in the atom in whose electron shell the ninth one “arrives” last d-electron. Indeed, the copper atom has 3 d-sublevel has 10 electrons, and 4 s- only one sublevel (Fig. 6.16 b).
The decrease in the energy of the orbitals of a fully or half-filled sublevel causes a number of important chemical phenomena, some of which you will become familiar with.
6.7. Outer and valence electrons, orbitals and sublevels
In chemistry, the properties of isolated atoms, as a rule, are not studied, since almost all atoms, when included in various substances, form chemical bonds. Chemical bonds are formed by the interaction of electron shells of atoms. For all atoms (except hydrogen), not all electrons take part in the formation of chemical bonds: boron has three out of five electrons, carbon has four out of six, and, for example, barium has two out of fifty-six. These "active" electrons are called valence electrons.
Valence electrons are sometimes confused with external electrons, but this is not the same thing.
Electronic clouds of outer electrons have a maximum radius (and a maximum value of the principal quantum number).

It is the outer electrons that take part in the formation of bonds in the first place, if only because when atoms approach each other, the electron clouds formed by these electrons come into contact first of all. But along with them, some electrons can also take part in the formation of a bond. pre-external(penultimate) layer, but only if they have an energy not very different from the energy of the outer electrons. Both electrons of an atom are valence electrons. (In lanthanides and actinides, even some “outer” electrons are valence)
The energy of valence electrons is much greater than the energy of other electrons of the atom, and valence electrons differ significantly less in energy from each other.
Outer electrons are always valence electrons only if the atom can form chemical bonds at all. Thus, both electrons of the helium atom are external, but they cannot be called valence, since the helium atom does not form any chemical bonds at all.
Valence electrons occupy valence orbitals, which in turn form valence sublevels.

As an example, consider an iron atom, the electronic configuration of which is shown in Fig. 6.17. Of the electrons of an iron atom, the maximum principal quantum number ( n= 4) have only two 4 s-electron. Consequently, they are the outer electrons of this atom. The outer orbitals of the iron atom are all orbitals with n= 4, and the outer sublevels are all the sublevels formed by these orbitals, that is, 4 s-,
4p-, 4d- and 4 f-EPU.
Outer electrons are always valence electrons, therefore 4 s-electrons of the iron atom are valence electrons. And if so, then 3 d-electrons with slightly higher energy will also be valence electrons. At the external level of the iron atom, in addition to the filled 4 s-AO there are still 4 free p-, 4d- and 4 f-AO. All of them are external, but only 4 of them are valence R-AO, since the energy of the remaining orbitals is much higher, and the appearance of electrons in these orbitals is not beneficial for the iron atom.
So, the iron atom
external electronic level – fourth,
external sublevels – 4 s-, 4p-, 4d- and 4 f-EPU,
outer orbitals – 4 s-, 4p-, 4d- and 4 f-AO,
outer electrons – two 4 s-electron (4 s 2),
outer electronic layer – fourth,
external electron cloud – 4 s-EO
valence sublevels – 4 s-, 4p-, and 3 d-EPU,
valence orbitals – 4 s-, 4p-, and 3 d-AO,
valence electrons – two 4 s-electron (4 s 2) and six 3 d-electrons (3 d 6).
Valence sublevels can be filled partially or completely with electrons, or they can remain completely free. As the nuclear charge increases, the energy values of all sublevels decrease, but due to the interaction of electrons with each other, the energy of different sublevels decreases at different “speeds.” Energy fully filled d- And f-sublevels decreases so much that they cease to be valence.
As an example, consider the atoms of titanium and arsenic (Fig. 6.18).

In the case of titanium atom 3 d-EPU is only partially filled with electrons, and its energy is greater than energy 4 s-EPU, and 3 d-electrons are valence. The arsenic atom has 3 d-EPU is completely filled with electrons, and its energy is significantly less than the energy of 4 s-EPU, and therefore 3 d-electrons are not valence.
In the examples given, we analyzed valence electron configuration titanium and arsenic atoms.
The valence electronic configuration of an atom is depicted as valence electron formula, or in the form energy diagram of valence sublevels.
VALENCE ELECTRONS, EXTERNAL ELECTRONS, VALENCE EPU, VALENCE AO, VALENCE ELECTRON CONFIGURATION OF AN ATOM, VALENCE ELECTRON FORMULA, VALENCE SUBLEVELS DIAGRAM.
1. On the energy diagrams you have compiled and in the complete electronic formulas of the atoms Na, Mg, Al, Si, P, S, Cl, Ar, indicate the outer and valence electrons. Write the valence electronic formulas of these atoms. On the energy diagrams, highlight the parts corresponding to the energy diagrams of the valence sublevels.
2. What do the electronic configurations of atoms have in common: a) Li and Na, B and Al, O and S, Ne and Ar; b) Zn and Mg, Sc and Al, Cr and S, Ti and Si; c) H and He, Li and O, K and Kr, Sc and Ga. What are their differences
3. How many valence sublevels are in the electron shell of an atom of each element: a) hydrogen, helium and lithium, b) nitrogen, sodium and sulfur, c) potassium, cobalt and germanium
4. How many valence orbitals are completely filled in the a) boron, b) fluorine, c) sodium atom?
5. How many orbitals with an unpaired electron does an atom have: a) boron, b) fluorine, c) iron
6. How many free outer orbitals does the manganese atom have? How many free valences?
7.For the next lesson, prepare a strip of paper 20 mm wide, divide it into cells (20 × 20 mm), and apply a natural series of elements (from hydrogen to meitnerium) to this strip.
8.In each cell, place the symbol of the element, its atomic number and valence electron formula, as shown in Fig. 6.19 (use Appendix 4).

6.8. Systematization of atoms according to the structure of their electron shells
The systematization of chemical elements is based on the natural series of elements
And principle of similarity of electron shells their atoms.
You are already familiar with the natural series of chemical elements. Now let's get acquainted with the principle of similarity of electronic shells.
Considering the valence electronic formulas of atoms in the ERE, it is easy to discover that for some atoms they differ only in the values of the principal quantum number. For example, 1 s 1 for hydrogen, 2 s 1 for lithium, 3 s 1 for sodium, etc. Or 2 s 2 2p 5 for fluorine, 3 s 2 3p 5 for chlorine, 4 s 2 4p 5 for bromine, etc. This means that the outer regions of the clouds of valence electrons of such atoms are very similar in shape and differ only in size (and, of course, electron density). And if so, then the electron clouds of such atoms and the corresponding valence configurations can be called similar. For atoms of different elements with similar electronic configurations we can write general valence electronic formulas: ns 1 in the first case and ns 2 n.p. 5 in the second. As you move through the natural series of elements, you can find other groups of atoms with similar valence configurations.
Thus, atoms with similar valence electron configurations are regularly found in the natural series of elements.
This is the principle of similarity of electronic shells.
Let's try to identify the type of this regularity. To do this, we will use the natural series of elements you made.

The ERE begins with hydrogen, the valence electronic formula of which is 1 s 1 . In search of similar valence configurations, we cut the natural series of elements in front of elements with a common valence electronic formula ns 1 (i.e. before lithium, before sodium, etc.). We received the so-called "periods" of the elements. Let's add the resulting “periods” so that they become table rows (see Fig. 6.20). As a result, only atoms in the first two columns of the table will have similar electronic configurations.
Let's try to achieve similarity of valence electronic configurations in other columns of the table. To do this, we cut out from the 6th and 7th periods elements with numbers 58 – 71 and 90 –103 (they fill 4 f- and 5 f-sublevels) and place them under the table. We will move the symbols of the remaining elements horizontally as shown in the figure. After this, the atoms of elements located in the same column of the table will have similar valence configurations, which can be expressed by general valence electronic formulas: ns 1 , ns 2 , ns 2 (n–1)d 1 , ns 2 (n–1)d 2 and so on until ns 2 n.p. 6. All deviations from the general valence formulas are explained by the same reasons as in the case of chromium and copper (see paragraph 6.6).
As you can see, by using the ERE and applying the principle of similarity of electron shells, we were able to systematize chemical elements. Such a system of chemical elements is called natural, since it is based solely on the laws of Nature. The table we received (Fig. 6.21) is one of the ways to graphically depict a natural system of elements and is called long-period table of chemical elements.

PRINCIPLE OF SIMILARITY OF ELECTRON SHELLS, NATURAL SYSTEM OF CHEMICAL ELEMENTS ("PERIODIC" SYSTEM), TABLE OF CHEMICAL ELEMENTS.
6.9. Long period table of chemical elements
Let's take a closer look at the structure of the long-period table of chemical elements.
The rows of this table, as you already know, are called "periods" of elements. The periods are numbered with Arabic numerals from 1 to 7. The first period has only two elements. The second and third periods, containing eight elements each, are called short periods. The fourth and fifth periods, containing 18 elements each, are called long periods. The sixth and seventh periods, containing 32 elements each, are called extra long periods.
The columns of this table are called groups elements. Group numbers are indicated by Roman numerals with Latin letters A or B.
Elements of some groups have their own common (group) names: elements of group IA (Li, Na, K, Rb, Cs, Fr) - alkaline elements(or alkali metal elements); Group IIA elements (Ca, Sr, Ba and Ra) – alkaline earth elements(or alkaline earth metal elements)(the name "alkali metals" and alkaline earth metals" refer to simple substances formed by the corresponding elements and should not be used as names of groups of elements); elements VIA group (O, S, Se, Te, Po) – chalcogens, group VIIA elements (F, Cl, Br, I, At) – halogens, group VIII elements (He, Ne, Ar, Kr, Xe, Rn) – noble gas elements.(The traditional name "noble gases" also refers to simple substances)
The elements with serial numbers 58 – 71 (Ce – Lu) usually placed at the bottom of the table are called lanthanides(“following lanthanum”), and elements with serial numbers 90 – 103 (Th – Lr) – actinides("following sea anemone"). There is a version of the long-period table, in which lanthanides and actinides are not cut out from the ERE, but remain in their places in ultra-long periods. This table is sometimes called ultra-long-period.
The long period table is divided into four block(or sections).
s-Block includes elements of IA and IIA groups with common valence electronic formulas ns 1 and ns 2
(s-elements).
r-Block includes elements from Group IIIA to VIIIA with common valence electronic formulas from ns 2 n.p. 1 to ns 2 n.p. 6 (p-elements).
d-Block includes elements from group IIIB to IIB with common valence electronic formulas from ns 2 (n–1)d 1 to ns 2 (n–1)d 10 (d-elements).
f-Block includes lanthanides and actinides ( f-elements).

Elements s- And p-blocks form A-groups, and elements d-block – B-group of the system of chemical elements. All f-elements are formally included in group IIIB.
The elements of the first period - hydrogen and helium - are s-elements and can be placed in groups IA and IIA. But helium is more often placed in group VIIIA as the element with which the period ends, which fully corresponds to its properties (helium, like all other simple substances formed by the elements of this group, is a noble gas). Hydrogen is often placed in group VIIA, since its properties are much closer to halogens than to alkaline elements.
Each of the periods of the system begins with an element having a valence configuration of atoms ns 1, since it is from these atoms that the formation of the next electronic layer begins, and ends with an element with a valence configuration of atoms ns 2 n.p. 6 (except for the first period). This makes it easy to identify on the energy diagram groups of sublevels filled with electrons in atoms of each period (Fig. 6.22). Do this work with all the sublevels shown in the copy you made of Figure 6.4. The sublevels highlighted in Figure 6.22 (except for completely filled d- And f-sublevels) are valence for atoms of all elements of a given period.
Appearance in periods s-, p-, d- or f-elements fully correspond to the filling sequence s-, p-, d- or f-sublevels with electrons. This feature of the system of elements allows, knowing the period and group into which a given element belongs, to immediately write down its valence electronic formula.
LONG-PERIOD TABLE OF CHEMICAL ELEMENTS, BLOCKS, PERIODS, GROUPS, ALKALINE ELEMENTS, ALKALINE EARTH ELEMENTS, CHALCOGENS, HALOGENS, NOBLE GASE ELEMENTS, LANTANOIDES, ACTINOIDS.
Write down the general valence electronic formulas of atoms of elements of a) IVA and IVB groups, b) IIIA and VIIB groups?
2. What do the electronic configurations of atoms of elements of groups A and B have in common? How are they different?
3. How many groups of elements are included in a) s-block, b) R-block, c) d-block?
4.Continue Figure 30 in the direction of increasing the energy of the sublevels and highlight groups of sublevels filled with electrons in the 4th, 5th and 6th periods.
5. List the valence sublevels of a) calcium, b) phosphorus, c) titanium, d) chlorine, e) sodium atoms. 6. State how s-, p- and d-elements differ from each other.
7.Explain why the membership of an atom in any element is determined by the number of protons in the nucleus, and not by the mass of this atom.
8.For atoms of lithium, aluminum, strontium, selenium, iron and lead, compose valence, full and abbreviated electronic formulas and draw energy diagrams of valence sublevels. 9.Which element atoms correspond to the following valence electronic formulas: 3 s 1 , 4s 1 3d 1 , 2s 2 2 p 6 , 5s 2 5p 2 , 5s 2 4d 2 ?
6.10. Types of electronic formulas of the atom. Algorithm for their compilation
For different purposes, we need to know either the total or valence configuration of an atom. Each of these electron configurations can be represented by either a formula or an energy diagram. That is, full electron configuration of an atom is expressed full electronic formula of an atom, or complete energy diagram of an atom. In its turn, valence electron configuration of an atom is expressed valence(or as it is often called, " short") electronic formula of the atom, or diagram of valence sublevels of an atom(Fig. 6.23).

Previously, we made electronic formulas for atoms using the atomic numbers of the elements. At the same time, we determined the sequence of filling sublevels with electrons according to the energy diagram: 1 s, 2s,
2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p,
6s, 4f, 5d, 6p, 7s and so on. And only by writing down the complete electronic formula could we write down the valence formula.
It is more convenient to write the valence electronic formula of an atom, which is most often used, based on the position of the element in the system of chemical elements, using period-group coordinates.
Let's take a closer look at how this is done for elements s-, p- And d-blocks
For elements s-block valence electronic formula of an atom consists of three symbols. In general, it can be written as follows:
In the first place (in place of the large cell) the period number is placed (equal to the main quantum number of these s-electrons), and on the third (in superscript) - the group number (equal to the number of valence electrons). Taking the magnesium atom (3rd period, group IIA) as an example, we get:
For elements p-block valence electronic formula of an atom consists of six symbols:
![]()
Here, in place of the large cells, the period number is also placed (equal to the main quantum number of these s- And p-electrons), and the group number (equal to the number of valence electrons) turns out to be equal to the sum of the superscripts. For the oxygen atom (2nd period, VIA group) we get:
2s 2 2p 4 .
Valence electronic formula of most elements d-block can be written like this:
As in previous cases, here instead of the first cell the period number is put (equal to the main quantum number of these s-electrons). The number in the second cell turns out to be one less, since the main quantum number of these d-electrons. The group number here is also equal to the sum of the indices. Example – valence electronic formula of titanium (4th period, IVB group): 4 s 2 3d 2 .
The group number is equal to the sum of the indices for the elements of the VIB group, but, as you remember, in their valence s-sublevel has only one electron, and the general valence electronic formula is ns 1 (n–1)d 5 . Therefore, the valence electronic formula, for example, of molybdenum (5th period) is 5 s 1 4d 5 .
It is also easy to compose the valence electronic formula of any element of the IB group, for example, gold (6th period)>–>6 s 1 5d 10, but in this case you need to remember that d- the electrons of the atoms of the elements of this group still remain valence, and some of them can participate in the formation of chemical bonds.
The general valence electronic formula of atoms of group IIB elements is ns 2 (n – 1)d 10 . Therefore, the valence electronic formula, for example, of a zinc atom is 4 s 2 3d 10 .
General rules The valence electronic formulas of the elements of the first triad (Fe, Co and Ni) also obey. Iron, an element of group VIIIB, has a valence electronic formula of 4 s 2 3d 6. The cobalt atom has one d-electron more (4 s 2 3d 7), and for the nickel atom - by two (4 s 2 3d 8).
Using only these rules for writing valence electronic formulas, it is impossible to compose electronic formulas for the atoms of some d-elements (Nb, Ru, Rh, Pd, Ir, Pt), since in them, due to the desire for highly symmetrical electron shells, the filling of valence sublevels with electrons has some additional features.
Knowing the valence electronic formula, you can write down the full electronic formula of the atom (see below).
Often, instead of cumbersome complete electronic formulas, they write abbreviated electronic formulas atoms. To compile them in the electronic formula, all the electrons of the atom except the valence ones are isolated, their symbols are placed in square brackets, and the part of the electronic formula corresponding to the electronic formula of the atom of the last element of the previous period (the element forming a noble gas) is replaced with the symbol of this atom.
Examples of electronic formulas of different types are given in Table 14.
Table 14. Examples of electronic formulas of atoms
Electronic formulas |
|||
Abbreviated |
Valence |
||
1s 2 2s 2 2p 3 |
2s 2 2p 3 |
2s 2 2p 3 |
|
1s 2 2s 2 2p 6 3s 2 3p 5 |
3s 2 3p 5 |
3s 2 3p 5 |
|
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 |
4s 2 3d 5 |
4s 2 3d 5 |
|
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3 |
4s 2 4p 3 |
4s 2 4p 3 |
|
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 |
4s 2 4p 6 |
4s 2 4p 6 |
|
Algorithm for compiling electronic formulas of atoms (using the example of the iodine atom)
№ |
Operation |
Result |
|
Determine the coordinates of the atom in the table of elements. |
Period 5, group VIIA |
||
Write the valence electron formula. |
5s 2 5p 5 |
||
Complete the symbols for the inner electrons in the order in which they fill the sublevels. |
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5 |
||
Considering the decrease in energy of fully filled d- And f-sublevels, write down the complete electronic formula. |
|
||
Label the valence electrons. |
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5 |
||
Identify the electron configuration of the preceding noble gas atom. |
|||
Write down the abbreviated electronic formula by combining everything in square brackets nonvalent electrons. |
5s 2 5p 5 |
Notes
1. For elements of the 2nd and 3rd periods, the third operation (without the fourth) immediately leads to the complete electronic formula.
2. (n – 1)d 10 -Electrons remain valence on the atoms of group IB elements.
COMPLETE ELECTRONIC FORMULA, VALENCE ELECTRONIC FORMULA, SHORTENED ELECTRONIC FORMULA, ALGORITHM FOR COMPILING ELECTRONIC FORMULAS OF ATOMS.
1. Make up the valence electronic formula of an atom of the element a) the second period of the third A group, b) the third period of the second A group, c) the fourth period of the fourth A group.
2.Make abbreviated electronic formulas for the atoms of magnesium, phosphorus, potassium, iron, bromine and argon.
6.11. Short period table of chemical elements
Over the 100-plus years that have passed since the discovery of the natural system of elements, several hundred different tables have been proposed that graphically reflect this system. Of these, in addition to the long-period table, the most widespread is the so-called short-period table of elements by D. I. Mendeleev. A short-period table is obtained from a long-period table if the 4th, 5th, 6th and 7th periods are cut in front of the elements of the IB group, moved apart and the resulting rows are folded in the same way as we previously folded the periods. The result is shown in Figure 6.24.

Lanthanides and actinides are also placed below the main table here.
IN groups This table contains elements whose atoms same number of valence electrons regardless of what orbitals these electrons are in. Thus, the elements chlorine (a typical element forming a non-metal; 3 s 2 3p 5) and manganese (a metal-forming element; 4 s 2 3d 5), not having similar electron shells, fall here into the same seventh group. The need to distinguish such elements forces us to distinguish them in groups subgroups: main– analogues of the A-groups of the long-period table and side– analogues of B-groups. In Figure 34, the symbols of the elements of the main subgroups are shifted to the left, and the symbols of the elements of the secondary subgroups are shifted to the right.
True, this arrangement of elements in the table also has its advantages, because it is the number of valence electrons that primarily determines the valence capabilities of an atom.
The long-period table reflects the laws of the electronic structure of atoms, the similarities and patterns of changes in the properties of simple substances and compounds across groups of elements, the regular changes in a number of physical quantities characterizing atoms, simple substances and compounds throughout the entire system of elements, and much more. The short-period table is less convenient in this regard.
SHORT-PERIOD TABLE, MAIN SUBGROUPS, SIDE SUBGROUPS.
1. Convert the long-period table you constructed from a natural series of elements into a short-period table. Do the reverse conversion.
2. Is it possible to compile a general valence electronic formula for atoms of elements of one group of the short-period table? Why?
6.12. Atomic sizes. Orbital radii
.The atom has no clear boundaries. What is considered the size of an isolated atom? The nucleus of an atom is surrounded by an electron shell, and the shell consists of electron clouds. The size of the EO is characterized by a radius r eo. All clouds in the outer layer have approximately the same radius. Therefore, the size of an atom can be characterized by this radius. It is called orbital radius of the atom(r 0).
The values of the orbital radii of atoms are given in Appendix 5.
The radius of the EO depends on the charge of the nucleus and on the orbital in which the electron forming this cloud is located. Consequently, the orbital radius of an atom depends on these same characteristics.
Let's consider the electronic shells of hydrogen and helium atoms. In both the hydrogen atom and the helium atom, electrons are located at 1 s-AO, and their clouds would have the same size if the charges of the nuclei of these atoms were the same. But the charge on the nucleus of a helium atom is twice as large as the charge on the nucleus of a hydrogen atom. According to Coulomb's law, the force of attraction acting on each electron of a helium atom is twice the force of attraction of an electron to the nucleus of a hydrogen atom. Therefore, the radius of the helium atom must be much smaller than the radius of the hydrogen atom. This is true: r 0 (He) / r 0 (H) = 0.291 E / 0.529 E 0.55.
The lithium atom has an outer electron at 2 s-AO, that is, forms a cloud of the second layer. Naturally, its radius should be larger. Really: r 0 (Li) = 1.586 E.
The atoms of the remaining elements of the second period have outer electrons (and 2 s, and 2 p) are located in the same second electron layer, and the nuclear charge of these atoms increases with increasing atomic number. Electrons are more strongly attracted to the nucleus, and, naturally, the radii of the atoms decrease. We could repeat these arguments for atoms of elements of other periods, but with one clarification: the orbital radius decreases monotonically only when each of the sublevels is filled.
But if we ignore the details, the general nature of the change in the sizes of atoms in a system of elements is as follows: with an increase in the ordinal number in a period, the orbital radii of atoms decrease, and in a group they increase. The largest atom is a cesium atom, and the smallest is a helium atom, but of the atoms of elements that form chemical compounds (helium and neon do not form them), the smallest is a fluorine atom.
Most atoms of elements in the natural series after the lanthanides have orbital radii that are somewhat smaller than would be expected based on general laws. This is due to the fact that between lanthanum and hafnium in the system of elements there are 14 lanthanides, and, therefore, the charge of the nucleus of the hafnium atom is 14 e more than lanthanum. Therefore, the outer electrons of these atoms are attracted to the nucleus more strongly than they would be in the absence of lanthanides (this effect is often called “lanthanide contraction”).
Please note that when moving from atoms of group VIIIA elements to atoms of group IA elements, the orbital radius increases abruptly. Consequently, our choice of the first elements of each period (see § 7) turned out to be correct.
ORBITAL RADIUS OF AN ATOM, ITS CHANGE IN THE SYSTEM OF ELEMENTS.
1.According to the data given in Appendix 5, draw on graph paper a graph of the dependence of the orbital radius of an atom on the atomic number of the element for elements with Z from 1 to 40. The length of the horizontal axis is 200 mm, the length of the vertical axis is 100 mm.
2. How can you characterize the appearance of the resulting broken line?
6.13. Atomic ionization energy
If you give an electron in an atom additional energy (you will learn how this can be done in a physics course), then the electron can move to another AO, that is, the atom will end up in excited state. This state is unstable, and the electron will almost immediately return to its original state, and excess energy will be released. But if the energy imparted to the electron is large enough, the electron can completely break away from the atom, while the atom ionized, that is, turns into a positively charged ion ( cation). The energy required for this is called atomic ionization energy(E And).
It is quite difficult to remove an electron from a single atom and measure the energy required for this, so it is practically determined and used molar ionization energy(E and m).
Molar ionization energy shows what is the minimum energy required to remove 1 mole of electrons from 1 mole of atoms (one electron from each atom). This value is usually measured in kilojoules per mole. The values of the molar ionization energy of the first electron for most elements are given in Appendix 6.
How does the ionization energy of an atom depend on the position of the element in the system of elements, that is, how does it change in the group and period?
In its physical meaning, ionization energy is equal to the work that must be expended to overcome the force of attraction between an electron and an atom when moving an electron from an atom to an infinite distance from it.
Where q– electron charge, Q is the charge of the cation remaining after the removal of an electron, and r o is the orbital radius of the atom.
AND q, And Q– the quantities are constant, and we can conclude that the work of removing an electron A, and with it the ionization energy E and, are inversely proportional to the orbital radius of the atom.
By analyzing the values of the orbital radii of atoms of various elements and the corresponding ionization energy values given in Appendices 5 and 6, you can make sure that the relationship between these quantities is close to proportional, but differs somewhat from it. The reason that our conclusion does not agree very well with the experimental data is that we used a very crude model that did not take into account many important factors. But even this rough model allowed us to draw the correct conclusion that with increasing orbital radius the ionization energy of the atom decreases and, conversely, with decreasing radius it increases.
Since in a period with increasing atomic number the orbital radius of atoms decreases, the ionization energy increases. In a group, as the atomic number increases, the orbital radius of atoms, as a rule, increases, and the ionization energy decreases. The highest molar ionization energy is found in the smallest atoms, helium atoms (2372 kJ/mol), and of the atoms capable of forming chemical bonds, fluorine atoms (1681 kJ/mol). The smallest is for the largest atoms, cesium atoms (376 kJ/mol). In a system of elements, the direction of increasing ionization energy can be shown schematically as follows: 
In chemistry, it is important that ionization energy characterizes the tendency of an atom to give up “its” electrons: the higher the ionization energy, the less inclined the atom is to give up electrons, and vice versa.
EXCITED STATE, IONIZATION, CATION, IONIZATION ENERGY, MOLAR IONIZATION ENERGY, CHANGE IN IONIZATION ENERGY IN A SYSTEM OF ELEMENTS.
1. Using the data given in Appendix 6, determine how much energy must be expended to remove one electron from all sodium atoms with a total mass of 1 g.
2. Using the data given in Appendix 6, determine how many times more energy is needed to remove one electron from all sodium atoms weighing 3 g than from all potassium atoms of the same mass. Why does this ratio differ from the ratio of the molar ionization energies of the same atoms?
3.According to the data given in Appendix 6, plot the dependence of the molar ionization energy on the atomic number for elements with Z from 1 to 40. The dimensions of the graph are the same as in the assignment to the previous paragraph. Check whether this graph corresponds to the choice of “periods” of the system of elements.
6.14. Electron affinity energy
.The second most important energy characteristic of an atom is electron affinity energy(E With).
In practice, as in the case of ionization energy, the corresponding molar quantity is usually used - molar electron affinity energy().
Molar electron affinity energy shows the energy released when one mole of electrons is added to one mole of neutral atoms (one electron for each atom). Like molar ionization energy, this quantity is also measured in kilojoules per mole.
At first glance, it may seem that energy should not be released in this case, because an atom is a neutral particle, and there are no electrostatic forces of attraction between a neutral atom and a negatively charged electron. On the contrary, approaching an atom, an electron, it would seem, should be repelled by the same negatively charged electrons that form the electron shell. Actually this is not true. Remember if you have ever had to deal with atomic chlorine. Of course not. After all, it exists only at very high temperatures. Even the more stable molecular chlorine practically does not occur in nature; if necessary, it must be obtained using chemical reactions. And you have to deal with sodium chloride (table salt) constantly. After all, table salt is consumed every day by humans with food. And in nature it occurs quite often. But table salt contains chloride ions, that is, chlorine atoms that have added one “extra” electron. One of the reasons why chloride ions are so common is that chlorine atoms have a tendency to gain electrons, that is, when chloride ions are formed from chlorine atoms and electrons, energy is released.
One of the reasons for the release of energy is already known to you - it is associated with an increase in the symmetry of the electron shell of the chlorine atom during the transition to singly charged anion. At the same time, as you remember, energy 3 p-sublevel decreases. There are other more complex reasons.
Due to the fact that the value of electron affinity energy is influenced by several factors, the nature of the change in this quantity in a system of elements is much more complex than the nature of the change in ionization energy. You can verify this by analyzing the table given in Appendix 7. But since the value of this quantity is determined, first of all, by the same electrostatic interaction as the values of ionization energy, then its change in the system of elements (according to at least in A-groups) is in general similar to the change in ionization energy, that is, the energy of electron affinity in the group decreases, and in the period it increases. It is maximum for fluorine (328 kJ/mol) and chlorine (349 kJ/mol) atoms. The nature of the change in electron affinity energy in a system of elements resembles the nature of the change in ionization energy, that is, the direction of increase in electron affinity energy can be shown schematically as follows:
2.On the same scale along the horizontal axis as in previous tasks, construct a graph of the dependence of the molar energy of electron affinity on the atomic number for atoms of elements with Z from 1 to 40 using app 7.
3.Which physical meaning have negative electron affinity energies?
4. Why, of all the atoms of elements of the 2nd period, only beryllium, nitrogen and neon have negative values of the molar energy of electron affinity?
6.15. The tendency of atoms to lose and gain electrons
You already know that the tendency of an atom to give up its own electrons and to add others’ electrons depends on its energy characteristics (ionization energy and electron affinity energy). Which atoms are more inclined to give up their electrons, and which ones are more inclined to accept others?
To answer this question, let us summarize in Table 15 everything that we know about the change in these inclinations in the system of elements.
Table 15. Changes in the propensity of atoms to give up their own electrons and gain foreign electrons
If identical particles have the same quantum numbers, then their wave function is symmetric with respect to the permutation of particles. It follows that two identical fermions included in the same system cannot be in the same states, because for fermions the wave function must be antisymmetric. Summarizing the experimental data, W. Pauli formed principle exceptions , Whereby fermion systems occur in nature only in states,described by antisymmetric wave functions(quantum mechanical formulation of the Pauli principle).
From this position follows a simpler formulation of the Pauli principle, which was introduced by him in quantum theory(1925) even before the construction of quantum mechanics: in a system of identical fermions any two of them cannot simultaneously be in the same state . Note that the number of identical bosons in the same state is not limited.
Let us recall that the state of an electron in an atom is uniquely determined by the set four quantum numbers :
· main n ;
· orbital l ![]() , usually these states are designated 1 s, 2d, 3f;
, usually these states are designated 1 s, 2d, 3f;
magnetic();
· magnetic spin ().
The distribution of electrons in an atom occurs according to the Pauli principle, which can be formulated for an atom in its simplest form: the same atom cannot have more than one electron with the same set of four quantum numbers: n, l, , :
Z (n, l, , ) = 0 or 1,
Where Z (n, l, , ) - the number of electrons in a quantum state, described by a set of four quantum numbers: n, l. . . Thus, the Pauli principle states that two electrons ,bound in the same atom differ in meaning ,at least ,one quantum number .
The maximum number of electrons in states described by a set of three quantum numbers n, l And m, and differing only in the orientation of the electron spins is equal to:
| , | (8.2.1) |
because the spin quantum number can only take two values: 1/2 and –1/2.
The maximum number of electrons in states defined by two quantum numbers n And l:
| (8.2.2) |
In this case, the vector of the orbital angular momentum of the electron can take in space (2 l+ 1) different orientations (Fig. 8.1).

The maximum number of electrons in states determined by the value of the principal quantum number n, equals:
| (8.2.3) |
Collection of electrons in a multi-electron atom,having the same principal quantum number n,called electron shell or layer .
In each shell, electrons are distributed according to subshells , corresponding to this l.
Region of space,in which there is a high probability of detecting an electron, called subshell or orbital . The main types of orbitals are shown in Fig. 8.1.
Since the orbital quantum number takes values from 0 to , the number of subshells is equal to the ordinal number n shells. The number of electrons in a subshell is determined by the magnetic and magnetic spin quantum numbers: the maximum number of electrons in a subshell with a given l equals 2(2 l+ 1). Shell designations, as well as the distribution of electrons across shells and subshells are given in Table. 1.
Table 1
| Principal quantum number n |
|||||||||||||||
| Shell symbol |
|||||||||||||||
| Maximum number of electrons in shell |
|||||||||||||||
| Orbital quantum number l |
|||||||||||||||
| Subshell symbol |
|||||||||||||||
| Maximum number electrons in subshell |
|||||||||||||||
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0