The most common knowledge is about three states of aggregation: liquid, solid, gaseous; sometimes they remember plasma, less often liquid crystalline. Lately A list of 17 phases of matter, taken from the famous () Stephen Fry, has circulated on the Internet. Therefore, we will tell you about them in more detail, because... you should know a little more about matter, if only in order to better understand the processes occurring in the Universe.
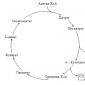
The list of aggregate states of matter given below increases from the coldest states to the hottest, etc. may be continued. At the same time, it should be understood that gaseous state(No. 11), the most “decompressed”, on both sides of the list the degree of compression of the substance and its pressure (with some reservations for such unstudied hypothetical states as quantum, beam or weakly symmetric) increase. After the text is a visual graph of phase transitions of matter.
1. Quantum- the state of aggregation of a substance, achieved when the temperature drops to absolute zero, as a result of which they disappear internal communications and matter crumbles into free quarks.
2. Bose-Einstein condensate- a state of aggregation of matter, the basis of which is bosons, cooled to temperatures close to absolute zero (less than a millionth of a degree above absolute zero). In such a very cool state, it is enough big number atoms find themselves in their minimum possible quantum states and quantum effects begin to manifest themselves at the macroscopic level. A Bose-Einstein condensate (often called a "Bose condensate" or simply "beck") occurs when you cool a chemical element to an extremely low temperatures(typically to just above absolute zero, minus 273 degrees Celsius, the theoretical temperature at which everything stops moving).
This is where completely strange things begin to happen to the substance. Processes usually observed only at the atomic level now occur on scales large enough to be observed with the naked eye. For example, if you place “back” in a laboratory beaker and provide the desired temperature, the substance will begin to creep up the wall and eventually come out on its own.
Apparently, here we are dealing with a futile attempt by a substance to lower its own energy (which is already at the lowest of all possible levels).
Slowing down atoms using cooling equipment produces a singular quantum state known as a Bose, or Bose-Einstein, condensate. This phenomenon was predicted in 1925 by A. Einstein, as a result of a generalization of the work of S. Bose, where statistical mechanics was built for particles ranging from massless photons to mass-bearing atoms (Einstein's manuscript, considered lost, was discovered in the library of Leiden University in 2005 ). The result of the efforts of Bose and Einstein was the Bose concept of a gas subject to Bose–Einstein statistics, which describes the statistical distribution of identical particles with integer spin called bosons. Bosons, which are, for example, individual elementary particles - photons, and entire atoms, can be in the same quantum states with each other. Einstein proposed that cooling boson atoms to very low temperatures would cause them to transform (or, in other words, condense) into the lowest possible quantum state. The result of such condensation will be the emergence new form substances.
This transition occurs below the critical temperature, which is for a homogeneous three-dimensional gas consisting of non-interacting particles without any internal degrees of freedom.
3. Fermion condensate- a state of aggregation of a substance, similar to backing, but different in structure. As they approach absolute zero, atoms behave differently depending on the magnitude of their own angular momentum (spin). Bosons have integer spins, while fermions have spins that are multiples of 1/2 (1/2, 3/2, 5/2). Fermions obey the Pauli exclusion principle, which states that no two fermions can have the same quantum state. There is no such prohibition for bosons, and therefore they have the opportunity to exist in one quantum state and thereby form the so-called Bose-Einstein condensate. The process of formation of this condensate is responsible for the transition to the superconducting state.
Electrons have spin 1/2 and are therefore classified as fermions. They combine into pairs (called Cooper pairs), which then form a Bose condensate.
American scientists have attempted to obtain a kind of molecules from fermion atoms by deep cooling. The difference from real molecules was that there was no chemical bond between the atoms - they simply moved together in a correlated manner. The bond between atoms turned out to be even stronger than between electrons in Cooper pairs. The resulting pairs of fermions have a total spin that is no longer a multiple of 1/2, therefore, they already behave like bosons and can form a Bose condensate with a single quantum state. During the experiment, a gas of potassium-40 atoms was cooled to 300 nanokelvins, while the gas was enclosed in a so-called optical trap. Then an external magnetic field was applied, with the help of which it was possible to change the nature of interactions between atoms - instead of strong repulsion, strong attraction began to be observed. When analyzing the influence of the magnetic field, it was possible to find a value at which the atoms began to behave like Cooper pairs of electrons. At the next stage of the experiment, scientists expect to obtain superconductivity effects for the fermion condensate.
4. Superfluid substance- a state in which a substance has virtually no viscosity, and during flow it does not experience friction with a solid surface. The consequence of this is, for example, such an interesting effect as the complete spontaneous “crawling out” of superfluid helium from the vessel along its walls against the force of gravity. Of course, there is no violation of the law of conservation of energy here. In the absence of frictional forces, helium is acted only by gravity forces, the forces of interatomic interaction between helium and the walls of the vessel and between helium atoms. So, the forces of interatomic interaction exceed all other forces combined. As a result, helium tends to spread as much as possible over all possible surfaces, and therefore “travels” along the walls of the vessel. In 1938, Soviet scientist Pyotr Kapitsa proved that helium can exist in a superfluid state.
It is worth noting that many of unusual properties helium have been known for quite some time. However, even in last years this chemical element spoils us with interesting and unexpected effects. Thus, in 2004, Moses Chan and Eun-Syong Kim from the University of Pennsylvania intrigued scientific world announcement that they had succeeded in obtaining a completely new state of helium - a superfluid solid. In this state, some helium atoms in the crystal lattice can flow around others, and helium can thus flow through itself. The “superhardness” effect was theoretically predicted back in 1969. And then in 2004 there seemed to be experimental confirmation. However, later and very interesting experiments showed that not everything is so simple, and perhaps this interpretation of the phenomenon, which was previously accepted as the superfluidity of solid helium, is incorrect.
The experiment of scientists led by Humphrey Maris from Brown University in the USA was simple and elegant. Scientists placed an upside-down test tube in a closed tank containing liquid helium. They froze part of the helium in the test tube and in the reservoir in such a way that the boundary between liquid and solid inside the test tube was higher than in the reservoir. In other words, in the upper part of the test tube there was liquid helium, in the lower part there was solid helium, it smoothly passed into the solid phase of the reservoir, above which a little liquid helium was poured - lower than the liquid level in the test tube. If liquid helium began to leak through solid helium, then the difference in levels would decrease, and then we can talk about solid superfluid helium. And in principle, in three of the 13 experiments, the difference in levels actually decreased.
5. Superhard substance- a state of aggregation in which matter is transparent and can “flow” like a liquid, but in fact it is devoid of viscosity. Such liquids have been known for many years; they are called superfluids. The fact is that if a superfluid is stirred, it will circulate almost forever, whereas a normal fluid will eventually calm down. The first two superfluids were created by researchers using helium-4 and helium-3. They were cooled to almost absolute zero - minus 273 degrees Celsius. And from helium-4, American scientists managed to obtain a supersolid body. They compressed frozen helium with more than 60 times the pressure, and then placed the glass filled with the substance on a rotating disk. At a temperature of 0.175 degrees Celsius, the disk suddenly began to spin more freely, which scientists say indicates that helium has become a superbody.
6. Solid- a state of aggregation of a substance, characterized by stability of shape and the nature of the thermal movement of atoms, which perform small vibrations around equilibrium positions. Steady state solids are crystalline. There are solids with ionic, covalent, metallic and other types of bonds between atoms, which determines their diversity physical properties. The electrical and some other properties of solids are mainly determined by the nature of the movement of the outer electrons of its atoms. Based on their electrical properties, solids are divided into dielectrics, semiconductors, and metals; based on their magnetic properties, solids are divided into diamagnetic, paramagnetic, and bodies with an ordered magnetic structure. Studies of the properties of solids have merged into a large field - physics. solid, the development of which is stimulated by the needs of technology.
7. Amorphous solid- a condensed state of aggregation of a substance, characterized by isotropy of physical properties due to the disordered arrangement of atoms and molecules. In amorphous solids, atoms vibrate around randomly located points. Unlike the crystalline state, the transition from solid amorphous to liquid occurs gradually. Various substances are in an amorphous state: glass, resins, plastics, etc.
8. Liquid crystal is a specific state of aggregation of a substance in which it simultaneously exhibits the properties of a crystal and a liquid. It should be noted right away that not all substances can be in a liquid crystalline state. However, some organic matter Having complex molecules, they can form a specific state of aggregation - liquid crystalline. This state occurs when crystals of certain substances melt. When they melt, a liquid crystalline phase is formed, which differs from ordinary liquids. This phase exists in the range from the melting temperature of the crystal to some higher temperature, when heated to which the liquid crystal turns into an ordinary liquid.
How does a liquid crystal differ from a liquid and an ordinary crystal and how is it similar to them? Like an ordinary liquid, a liquid crystal has fluidity and takes the shape of the container in which it is placed. This is how it differs from the crystals known to everyone. However, despite this property, which unites it with a liquid, it has a property characteristic of crystals. This is the ordering in space of the molecules that form the crystal. True, this ordering is not as complete as in ordinary crystals, but, nevertheless, it significantly affects the properties of liquid crystals, which distinguishes them from ordinary liquids. Incomplete spatial ordering of the molecules forming a liquid crystal is manifested in the fact that in liquid crystals there is no complete order in the spatial arrangement of the centers of gravity of molecules, although there may be partial order. This means that they do not have a rigid crystal lattice. Therefore, liquid crystals, like ordinary liquids, have the property of fluidity.
A mandatory property of liquid crystals, which brings them closer to ordinary crystals, is the presence of an order of spatial orientation of the molecules. This order in orientation can manifest itself, for example, in the fact that all the long axes of molecules in a liquid crystal sample are oriented in the same way. These molecules must have an elongated shape. In addition to the simplest named ordering of molecular axes, a more complex orientational order of molecules can occur in a liquid crystal.
Depending on the type of ordering of the molecular axes, liquid crystals are divided into three types: nematic, smectic and cholesteric.
Research on the physics of liquid crystals and their applications is currently being carried out on a wide front in all the most developed countries of the world. Domestic research is concentrated in both academic and industrial research institutions and has a long tradition. The works of V.K., completed back in the thirties in Leningrad, became widely known and recognized. Fredericks to V.N. Tsvetkova. In recent years, the rapid study of liquid crystals has seen domestic researchers also make a significant contribution to the development of the study of liquid crystals in general and, in particular, the optics of liquid crystals. Thus, the works of I.G. Chistyakova, A.P. Kapustina, S.A. Brazovsky, S.A. Pikina, L.M. Blinov and many other Soviet researchers are widely known to the scientific community and serve as the foundation for a number of effective technical applications of liquid crystals.
The existence of liquid crystals was established a long time ago, namely in 1888, that is, almost a century ago. Although scientists encountered this state of matter before 1888, it was officially discovered later.
The first to discover liquid crystals was the Austrian botanist Reinitzer. While studying the new substance cholesteryl benzoate he synthesized, he discovered that at a temperature of 145°C the crystals of this substance melt, forming a cloudy liquid that strongly scatters light. As heating continues, upon reaching a temperature of 179°C, the liquid becomes clear, i.e., it begins to behave optically like an ordinary liquid, for example water. Cholesteryl benzoate showed unexpected properties in the turbid phase. Examining this phase under a polarizing microscope, Reinitzer discovered that it exhibits birefringence. This means that the refractive index of light, i.e. the speed of light in this phase, depends on the polarization.
9. Liquid- a state of aggregation of a substance that combines the features solid state(volume conservation, certain tensile strength) and gaseous (shape variability). Liquids are characterized by short-range order in the arrangement of particles (molecules, atoms) and a small difference in the kinetic energy of thermal motion of molecules and their potential energy interactions. The thermal motion of liquid molecules consists of oscillations around equilibrium positions and relatively rare jumps from one equilibrium position to another; the fluidity of the liquid is associated with this.
10. Supercritical fluid(SCF) is a state of aggregation of a substance in which the difference between the liquid and gas phases disappears. Any substance at a temperature and pressure above its critical point is a supercritical fluid. The properties of a substance in the supercritical state are intermediate between its properties in the gas and liquid phases. Thus, SCF has a high density, close to a liquid, and low viscosity, like gases. The diffusion coefficient in this case has a value intermediate between liquid and gas. Substances in a supercritical state can be used as substitutes for organic solvents in laboratory and industrial processes. Supercritical water and supercritical carbon dioxide have received the greatest interest and distribution due to certain properties.
One of the most important properties of the supercritical state is the ability to dissolve substances. By changing the temperature or pressure of the fluid, you can change its properties over a wide range. Thus, it is possible to obtain a fluid whose properties are close to either a liquid or a gas. Thus, the dissolving ability of a fluid increases with increasing density (at a constant temperature). Since density increases with increasing pressure, changing the pressure can influence the dissolving ability of the fluid (at a constant temperature). In the case of temperature, the dependence of the properties of the fluid is somewhat more complex - at a constant density, the dissolving ability of the fluid also increases, but near the critical point, a slight increase in temperature can lead to a sharp drop in density, and, accordingly, the dissolving ability. Supercritical fluids mix with each other without limit, so when the critical point of the mixture is reached, the system will always be single-phase. The approximate critical temperature of a binary mixture can be calculated as the arithmetic mean of the critical parameters of the substances Tc(mix) = (mole fraction A) x TcA + (mole fraction B) x TcB.
11. Gaseous- (French gaz, from Greek chaos - chaos), the state of aggregation of a substance in which kinetic energy The thermal motion of its particles (molecules, atoms, ions) significantly exceeds the potential energy of interactions between them, and therefore the particles move freely, uniformly filling the entire volume provided to them in the absence of external fields.
12. Plasma- (from the Greek plasma - sculpted, shaped), a state of matter that is an ionized gas in which the concentrations of positive and negative charges are equal (quasi-neutrality). The vast majority of matter in the Universe is in the plasma state: stars, galactic nebulae and the interstellar medium. Near Earth, plasma exists in the form of the solar wind, magnetosphere and ionosphere. High-temperature plasma (T ~ 106 - 108K) from a mixture of deuterium and tritium is being studied with the aim of implementing controlled thermonuclear fusion. Low-temperature plasma (T Ј 105K) is used in various gas-discharge devices ( gas lasers, ion devices, MHD generators, plasmatrons, plasma engines, etc.), as well as in technology (see Plasma metallurgy, Plasma drilling, Plasma technology).
13. Degenerate matter— is an intermediate stage between plasma and neutronium. It is observed in white dwarfs and plays important role in the evolution of stars. When atoms are subjected to extremely high temperatures and pressures, they lose their electrons (they become electron gas). In other words, they are completely ionized (plasma). The pressure of such a gas (plasma) is determined by the pressure of the electrons. If the density is very high, all particles are forced closer to each other. Electrons can exist in states with specific energies, and no two electrons can have the same energy (unless their spins are opposite). Thus, in a dense gas, all lower energy levels are filled with electrons. Such a gas is called degenerate. In this state, electrons exhibit degenerate electron pressure, which counteracts the forces of gravity.
14. Neutronium- a state of aggregation into which matter passes at ultra-high pressure, which is still unattainable in the laboratory, but exists inside neutron stars. During the transition to the neutron state, the electrons of the substance interact with protons and turn into neutrons. As a result, matter in the neutron state consists entirely of neutrons and has a density on the order of nuclear. The temperature of the substance should not be too high (in energy equivalent, no more than a hundred MeV).
With a strong increase in temperature (hundreds of MeV and above), various mesons begin to be born and annihilate in the neutron state. With a further increase in temperature, deconfinement occurs, and the substance passes into the state of quark-gluon plasma. It no longer consists of hadrons, but of constantly being born and disappearing quarks and gluons.
15. Quark-gluon plasma(chromoplasm) - a state of aggregation of matter in high-energy physics and elementary particle physics, in which hadronic matter passes into a state similar to the state in which electrons and ions are found in ordinary plasma.
Typically, the matter in hadrons is in the so-called colorless (“white”) state. That is, quarks of different colors cancel each other out. A similar state exists in ordinary matter - when all atoms are electrically neutral, that is,
positive charges they are compensated by negative ones. At high temperatures ionization of atoms can occur, while the charges are separated, and the substance becomes, as they say, “quasi-neutral”. That is, the entire cloud of matter as a whole remains neutral, but its individual particles cease to be neutral. The same thing, apparently, can happen with hadronic matter - at very high energies, color is released and makes the substance “quasi-colorless.”
Presumably, the matter of the Universe was in a state of quark-gluon plasma in the first moments after the Big Bang. Now quark-gluon plasma can be formed for a short time during collisions of particles of very high energies.
Quark-gluon plasma was produced experimentally at the RHIC accelerator at Brookhaven National Laboratory in 2005. The maximum plasma temperature of 4 trillion degrees Celsius was obtained there in February 2010.
16. Strange substance- a state of aggregation in which matter is compressed to maximum density values; it can exist in the form of “quark soup”. Cubic centimeter substances in this state will weigh billions of tons; in addition, it will transform any normal substance it comes into contact with into the same “strange” form with the release of a significant amount of energy.
The energy that can be released when the star's core turns into "strange matter" will lead to a super-powerful explosion of a "quark nova" - and, according to Leahy and Uyed, this is exactly what astronomers observed in September 2006.
The process of formation of this substance began with an ordinary supernova, into which a massive star turned. As a result of the first explosion, a neutron star was formed. But according to Leahy and Uyed, it was very short-lived as its rotation seemed to be slowed down by its own magnetic field, it began to compress even more, with the formation of a clump of “strange matter,” which led to an even more powerful release of energy than during a normal supernova explosion - and the outer layers of matter of the former neutron star scattered into the surrounding space at a speed close to the speed of light .
17. Strongly symmetrical substance- this is a substance compressed to such an extent that the microparticles inside it are layered on top of each other, and the body itself collapses into a black hole. The term “symmetry” is explained as follows: Let’s take the aggregative states of matter known to everyone from school - solid, liquid, gaseous. For definiteness, let us consider an ideal infinite crystal as a solid. There is a certain, so-called discrete symmetry with respect to transfer. This means that if you move the crystal lattice by a distance equal to the interval between two atoms, nothing will change in it - the crystal will coincide with itself. If the crystal is melted, then the symmetry of the resulting liquid will be different: it will increase. In a crystal, only points remote from each other at certain distances, the so-called nodes of the crystal lattice, in which identical atoms were located, were equivalent.
The liquid is homogeneous throughout its entire volume, all its points are indistinguishable from one another. This means that liquids can be displaced by any arbitrary distances (and not just some discrete ones, as in a crystal) or rotated by any arbitrary angles (which cannot be done in crystals at all) and it will coincide with itself. Its degree of symmetry is higher. Gas is even more symmetrical: the liquid occupies a certain volume in the vessel and there is an asymmetry inside the vessel where there is liquid and points where it is not. Gas occupies the entire volume provided to it, and in this sense, all its points are indistinguishable from one another. Still, here it would be more correct to talk not about points, but about small, but macroscopic elements, because at the microscopic level there are still differences. At some points in this moment time there are atoms or molecules, but others do not. Symmetry is observed only on average, either over some macroscopic volume parameters or over time.
But there is still no instant symmetry at the microscopic level. If a substance is compressed very strongly, to pressures that are unacceptable in everyday life, compressed so that the atoms are crushed, their shells penetrate each other, and the nuclei begin to touch, symmetry arises at the microscopic level. All nuclei are identical and pressed against each other, there are not only interatomic, but also internuclear distances, and the substance becomes homogeneous (strange substance).
But there is also a submicroscopic level. Nuclei are made up of protons and neutrons that move around inside the nucleus. There is also some space between them. If you continue to compress so that the nuclei are crushed, the nucleons will press tightly against each other. Then, at the submicroscopic level, symmetry will appear, which does not exist even inside ordinary nuclei.
From what has been said, one can discern a very definite trend: the higher the temperature and the greater the pressure, the more symmetrical the substance becomes. Based on these considerations, a substance compressed to its maximum is called highly symmetrical.
18. Weakly symmetrical matter- a state opposite to strongly symmetrical matter in its properties, present in the very early Universe at a temperature close to Planck's, perhaps 10-12 seconds after the Big Bang, when the strong, weak and electromagnetic forces represented a single superforce. In this state, the substance is compressed to such an extent that its mass turns into energy, which begins to inflate, that is, expand indefinitely. It is not yet possible to achieve the energies for experimentally obtaining superpower and transferring matter into this phase under terrestrial conditions, although such attempts were made at the Large Hadron Collider to study the early universe. Due to the absence of gravitational interaction in the superforce that forms this substance, the superforce is not sufficiently symmetrical in comparison with the supersymmetric force containing all 4 types of interactions. Therefore, this state of aggregation received such a name.
19. Ray substance- this, in fact, is no longer matter at all, but energy in its pure form. However, it is precisely this hypothetical state of aggregation that a body that has reached the speed of light will take. It can also be obtained by heating the body to the Planck temperature (1032K), that is, accelerating the molecules of the substance to the speed of light. As follows from the theory of relativity, when a speed reaches more than 0.99 s, the mass of the body begins to grow much faster than with “normal” acceleration; in addition, the body elongates, heats up, that is, it begins to radiate in the infrared spectrum. When crossing the threshold of 0.999 s, the body changes radically and begins a rapid phase transition up to the ray state. As follows from Einstein’s formula, taken in its entirety, the growing mass of the final substance consists of masses separated from the body in the form of thermal, x-ray, optical and other radiation, the energy of each of which is described by the next term in the formula. Thus, a body that approaches the speed of light will begin to emit in all spectra, grow in length and slow down in time, thinning to the Planck length, that is, upon reaching speed c, the body will turn into an infinitely long and thin beam, moving at the speed of light and consisting of photons that have no length, and its infinite mass will be completely converted into energy. Therefore, such a substance is called ray.
“Alcohols” From history Did you know that back in the 4th century. BC e. did people know how to make drinks containing ethyl alcohol? Wine was produced by fermenting fruit and berry juices. However, they learned to extract the intoxicating component from it much later. In the 11th century alchemists captured vapors of a volatile substance that was released when wine was heated Definition Alcohols (obsolete alcohols) are organic compounds containing one or more hydroxyl groups (hydroxyl, OH) directly bonded to the carbon atom in the hydrocarbon radical General formula alcohols CxHy(OH)n General formula of monohydric saturated alcohols CnH2n+1OH Classification of alcohols Based on the number of hydroxyl groups CxHy(OH)n Monohydric alcohols CH3 - CH2 - CH2 OH Dihydric glycols CH3 - CH - CH2 OH OH Triatomic glycerols CH2 - CH - CH2 OH OH OH Classification of alcohols By the nature of the hydrocarbon hydrocarbon radical radical CxHy(OH)n CxHy(OH)n Limiting Limiting CH3 CH3 –– CH CH2 CH2 2 ––CH 2 OH OH Unsaturated Unsaturated CH CH2 = CH CH––CH CH2 2 = 2 OH OH Aromatic Aromatic CH CH2 OH 2 --OH Nomenclature of alcohols Look at the table and draw a conclusion about the nomenclature of alcohols NOMENCLATURE AND ISOMERICS When forming the names of alcohols, a (generic) suffix is added to the name of the hydrocarbon corresponding to the alcohol. The numbers after the suffix indicate the position of the hydroxyl group in the main chain: H | H- C – O H | H methanol H H H |3 |2 |1 H- C – C – C -OH | | | H H H propanol-1 H H H | 1 | 2 |3 H - C – C – C -H | | | H OH H propanol -2 TYPES OF ISOMERITY 1. Positional isomerism functional group(propanol–1 and propanol–2) 2. Isomerism of the carbon skeleton CH3-CH2-CH2-CH2-OH butanol-1 CH3-CH-CH2-OH | CH3 2-methylpropanol-1 3. Interclass isomerism - alcohols are isomeric to ethers: CH3-CH2-OH ethanol CH3-O-CH3 dimethyl ether Conclusion The names of monohydric alcohols are formed from the name of the hydrocarbon with the longest carbon chain containing a hydroxyl group by adding suffix -ol For polyhydric alcohols, before the suffix -ol in Greek (-di-, -tri-, ...) the number of hydroxyl groups is indicated For example: CH3-CH2-OH ethanol Types of isomerism of alcohols Structural 1. Carbon chain 2. Positions of the functional group PHYSICAL PROPERTIES Lower alcohols (C1-C11) are volatile liquids with a pungent odor Higher alcohols (C12- and higher) are solids with a pleasant odor PHYSICAL PROPERTIES Name Formula Pl. g/cm3 tpl.C tboil.C Methyl CH3OH 0.792 -97 64 Ethyl C2H5OH 0.790 -114 78 Propyl CH3CH2CH2OH 0.804 -120 92 Isopropyl CH3-CH(OH)-CH3 0.786 -88 82 Butyl CH3CH2CH2CH2OH 0, 810 -90 118 Features of physical properties: state of aggregation Methyl alcohol (the first representative homologous series alcohols) – liquid. Maybe he has a big one molecular mass? No. Much less than carbon dioxide. Then what is it? R – O … H – O …H – O H R R It turns out that it’s all about the hydrogen bonds that form between alcohol molecules and prevent individual molecules from flying away. Feature of physical properties: solubility in water Lower alcohols are soluble in water, higher alcohols are insoluble. Why? CH3 – O…H – O…N – O N H CH3 What if the radical is large? CH3 – CH2 – CH2 – CH2 – CH2 – O ... H – O H H Hydrogen bonds are too weak to hold an alcohol molecule, which has a large insoluble part, between water molecules Feature of physical properties: contraction Why is volume never used when solving calculation problems? but only by mass? Mix 500 ml of alcohol and 500 ml of water. We get 930 ml of solution. The hydrogen bonds between the molecules of alcohol and water are so strong that the total volume of the solution decreases, its “compression” (from the Latin contraktio - compression). Certain representatives of alcohols Monohydric alcohol - methanol Colorless liquid with a boiling point of 64C, a characteristic odor Lighter than water. Burns with a colorless flame. Used as a solvent and fuel in internal combustion engines Methanol is a poison The toxic effect of methanol is based on damage to the nervous and vascular system. Ingestion of 5-10 ml of methanol leads to severe poisoning, and 30 ml or more leads to death. Monohydric alcohol - ethanol Colorless liquid with a characteristic odor and burning taste, boiling point 78C. Lighter than water. Mixes with her in any relationship. Easily flammable, burns with a weakly glowing bluish flame. Friendship with the traffic police Are alcohols friends with the traffic police? But how! Have you ever been stopped by a traffic police inspector? Have you ever breathed into a tube? If you are unlucky, then an oxidation reaction of alcohol took place, during which the color changed, and you had to pay a fine Interesting question. Alcohol is a xenobiotic - a substance not found in human body, but affecting his life. It all depends on the dose. 1. Alcohol is a nutrient that provides the body with energy. In the Middle Ages, the body received about 25% of its energy through alcohol consumption; 2. Alcohol is medicine, which has a disinfectant and antibacterial effect; 3. Alcohol is a poison that disrupts natural biological processes , which destroys internal organs and the psyche and, if consumed excessively, leads to death. Use of ethanol Ethyl alcohol is used in the preparation of various alcoholic beverages; In medicine for the preparation of extracts from medicinal plants, as well as for disinfection; In cosmetics and perfumery, ethanol is a solvent for perfumes and lotions. Harmful effects of ethanol At the beginning of intoxication, the structures of the cerebral cortex suffer; the activity of the brain centers that control behavior is suppressed: rational control over actions is lost, and the critical attitude towards oneself decreases. I. P. Pavlov called this condition “a riot of the subcortex” With a very high alcohol content in the blood, the activity of the motor centers of the brain is inhibited, the function of the cerebellum is mainly affected - the person completely loses orientation Harmful effects of ethanol Changes in the structure of the brain caused by many years of alcohol intoxication, almost are irreversible, and even after prolonged abstinence from drinking alcohol, they persist. If a person cannot stop, then organic and, therefore, mental deviations from the norm increase. Harmful effects of ethanol Alcohol has an extremely adverse effect on the blood vessels of the brain. At the beginning of intoxication, they expand, blood flow in them slows down, which leads to congestion in the brain. Then, when in addition to alcohol, harmful products of its incomplete breakdown begin to accumulate in the blood, a sharp spasm occurs, vasoconstriction occurs, and dangerous complications develop, such as cerebral strokes, leading to severe disability and even death. QUESTIONS FOR REVISION 1. 2. 3. 4. 5. 6. 7. 8. One container without a label contains water, and the other contains alcohol. Is it possible to use an indicator to recognize them? Who owns the honor of obtaining pure alcohol? Can alcohol be a solid? The molecular weight of methanol is 32, and carbon dioxide is 44. Draw a conclusion about the state of aggregation of alcohol. Mix a liter of alcohol and a liter of water. Determine the volume of the mixture. How to deceive a traffic police inspector? Can anhydrous absolute alcohol give off water? What are xenobiotics and how do they relate to alcohols? ANSWERS 1. 2. 3. 4. 5. 6. 7. 8. It’s impossible. The indicators do not affect alcohols and their aqueous solutions. Of course, alchemists. Maybe if this alcohol contains 12 carbon atoms or more. No conclusion can be drawn from these data. Hydrogen bonds between alcohol molecules, given the low molecular weight of these molecules, make the boiling point of alcohol abnormally high. The volume of the mixture will not be two liters, but much smaller, approximately 1 liter - 860 ml. Don't drink while driving. Maybe if you heat it up and add conc. sulfuric acid. Don’t be lazy and remember everything you heard about alcohol, decide for yourself once and for all what your dose is……. and is it needed at all????? Polyhydric alcohol ethylene glycol Ethylene glycol is a representative of saturated dihydric alcohols - glycols; The name glycols was given due to the sweet taste of many representatives of the series (Greek “glycos” - sweet); Ethylene glycol is a syrupy liquid with a sweet taste, odorless, and poisonous. Mixes well with water and alcohol, hygroscopic Application of ethylene glycol An important property of ethylene glycol is the ability to lower the freezing point of water, which is why the substance is widely used as a component of automobile antifreezes and antifreeze liquids; It is used to produce lavsan (valuable synthetic fiber) Ethylene glycol is a poison Doses causing fatal ethylene glycol poisoning vary widely - from 100 to 600 ml. According to a number of authors, the lethal dose for humans is 50-150 ml. The mortality rate due to ethylene glycol is very high and accounts for more than 60% of all cases of poisoning; The mechanism of the toxic effect of ethylene glycol has not been sufficiently studied to date. Ethylene glycol is quickly absorbed (including through the pores of the skin) and circulates in the blood unchanged for several hours, reaching its maximum concentration after 2-5 hours. Then its content in the blood gradually decreases, and it is fixed in the tissues. Polyhydric alcohol glycerin Glycerin is a trihydric saturated alcohol. Colorless, viscous, hygroscopic, sweet-tasting liquid. Miscible with water in any ratio, a good solvent. Reacts with nitric acid to form nitroglycerin. With carboxylic acids it forms fats and oils CH2 – CH – CH2 OH OH OH Applications of glycerin Used in production of nitroglycerin explosives; When processing leather; As a component of some adhesives; In the production of plastics, glycerin is used as a plasticizer; In the production of confectionery and beverages (as a food additive E422) Qualitative reaction to polyhydric alcohols Qualitative reaction to polyhydric alcohols The reaction to polyhydric alcohols is their interaction with a freshly obtained precipitate of copper (II) hydroxide, which dissolves to form a bright blue-violet solution Tasks Fill in work card for the lesson; Answer the test questions; Solve the crossword puzzle Worksheet for the lesson “Alcohols” General formula of alcohols Name the substances: CH3OH CH3-CH2-CH2-CH2-OH CH2(OH)-CH2(OH) Write the structural formula of propanol-2 What is the definition of atomicity of alcohol? List the applications of ethanol What alcohols are used in the food industry? What alcohol causes fatal poisoning when 30 ml enters the body? What substance is used as an antifreeze liquid? How to distinguish polyhydric alcohol from monohydric alcohol? Preparation methods Laboratory Hydrolysis of haloalkanes: R-CL+NaOH R-OH+NaCL Hydration of alkenes: CH2=CH2+H2O C2H5OH Hydrogenation of carbonyl compounds Industrial Synthesis of methanol from synthesis gas CO+2H2 CH3-OH (at elevated pressure, high temperature and zinc oxide catalyst) Hydration of alkenes Fermentation of glucose: C6H12O6 2C2H5OH+2CO2 Chemical properties I. Reactions with rupture of the RO–H bond Alcohols react with alkali and alkaline earth metals, forming salt-like compounds - alcoholates 2СH CH CH OH + 2Na 2CH CH CH ONa + H 2CH CH OH + Ca (CH CH O) Ca + H 3 2 3 2 2 3 3 2 2 2 2 2 2 Interaction with organic acids (esterification reaction) leads to the formation of esters. CH COОH + HOC H CH COОC H (ethyl acetate (ethyl acetate)) + H O 3 2 5 3 2 5 2 II. Reactions involving breaking the R–OH bond With hydrogen halides: R–OH + HBr R–Br + H2O III. Oxidation reactions Alcohols burn: 2С3H7ОH + 9O2 6СO2 + 8H2O Under the action of oxidizing agents: primary alcohols are converted into aldehydes, secondary alcohols into ketones IV. Dehydration Occurs when heated with water-removing reagents (conc. H2SO4). 1. Intramolecular dehydration leads to the formation of alkenes CH3–CH2–OH CH2=CH2 + H2O 2. Intermolecular dehydration gives ethers R-OH + H-O–R R–O–R(ether) + H2O
All substances can be in different states of aggregation - solid, liquid, gaseous and plasma. In ancient times it was believed that the world consists of earth, water, air and fire. The aggregate states of substances correspond to this visual division. Experience shows that the boundaries between states of aggregation are very arbitrary. Gases at low pressures and low temperatures are considered ideal, the molecules in them correspond material points that can only collide according to laws elastic impact. The forces of interaction between molecules at the moment of impact are negligible, and the collisions themselves occur without loss of mechanical energy. But as the distance between molecules increases, the interaction of molecules must also be taken into account. These interactions begin to affect the transition from a gaseous state to a liquid or solid. Various types of interactions can occur between molecules.
The forces of intermolecular interaction are not saturable, differing from the forces chemical interaction atoms leading to the formation of molecules. They can be electrostatic due to interactions between charged particles. Experience has shown that quantum mechanical interaction, which depends on the distance and mutual orientation of molecules, is negligible at distances between molecules of more than 10 -9 m. In rarefied gases it can be neglected or it can be assumed that the potential interaction energy is practically equal to zero. At short distances this energy is small, and mutual attractive forces act
at - mutual repulsion and force
attraction and repulsion of molecules are balanced and F= 0. Here the forces are determined by their connection with potential energy. But the particles move, possessing a certain reserve of kinetic energy.
gii. Let one molecule be motionless, and another collide with it, having such a supply of energy. As the molecules approach each other, the attractive forces do positive work and the potential energy of their interaction decreases to a distance. At the same time, the kinetic energy (and speed) increases. When the distance becomes less, the attractive forces will be replaced by repulsive forces. The work done by the molecule against these forces is negative.
The molecule will move closer to a stationary molecule until its kinetic energy is completely converted into potential. Minimum distance d, the distance at which molecules can approach is called effective diameter of the molecule. After stopping, the molecule will begin to move away under the influence of repulsive forces with increasing speed. Having passed the distance again, the molecule will fall into the region of attractive forces, which will slow down its removal. The effective diameter depends on the initial reserve of kinetic energy, i.e. this value is not constant. At distances equal to each other, the potential energy of interaction is infinite great importance or a “barrier” that prevents the centers of molecules from approaching a smaller distance. The ratio of the average potential interaction energy to the average kinetic energy determines the state of aggregation of a substance: for gases, for liquids, for solids
Condensed matter includes liquids and solids. In them, atoms and molecules are located close, almost touching. The average distance between the centers of molecules in liquids and solids is of the order of (2 -5) 10 -10 m. Their densities are also approximately the same. Interatomic distances exceed the distances at which electron clouds penetrate each other so much that repulsive forces arise. For comparison, in gases under normal conditions the average distance between molecules is about 33 10 -10 m.
IN liquids intermolecular interaction has a stronger effect, thermal movement molecules manifest themselves in weak vibrations around the equilibrium position and even jumps from one position to another. Therefore, they only have short-range order in the arrangement of particles, that is, consistency in the arrangement of only the nearest particles, and characteristic fluidity.
Solids They are characterized by structural rigidity, have a precisely defined volume and shape, which change much less under the influence of temperature and pressure. In solids, amorphous and crystalline states are possible. There are also intermediate substances - liquid crystals. But atoms in solids are not at all stationary, as one might think. Each of them fluctuates all the time under the influence of elastic forces arising between its neighbors. Most elements and compounds have a crystalline structure under a microscope.
Thus, table salt grains look like perfect cubes. In crystals, atoms are fixed at the sites of the crystal lattice and can vibrate only near the lattice sites. Crystals constitute true solids, and solids such as plastic or asphalt occupy an intermediate position between solids and liquids. Amorphous body has, like a liquid, short-range order, but the probability of jumps is low. Thus, glass can be considered as a supercooled liquid with increased viscosity. Liquid crystals have the fluidity of liquids, but retain the orderly arrangement of atoms and have anisotropy of properties.
Chemical bonds atoms (ions) in crystals are the same as in molecules. The structure and rigidity of solids are determined by differences in the electrostatic forces that bind together the atoms that make up the body. The mechanism that binds atoms into molecules can lead to the formation of solid periodic structures that can be considered as macromolecules. Like ionic and covalent molecules, there are ionic and covalent crystals. Ionic lattices in crystals are held together by ionic bonds (see Fig. 7.1). The structure of table salt is such that each sodium ion has six neighbors - chlorine ions. This distribution corresponds to a minimum energy, i.e., when such a configuration is formed, the maximum energy is released. Therefore, as the temperature drops below the melting point, there is a tendency to form pure crystals. As the temperature rises, the thermal kinetic energy is sufficient to break the bond, the crystal will begin to melt, and the structure will begin to collapse. Crystal polymorphism is the ability to form states with different crystal structures.
When distribution electric charge changes in neutral atoms, weak interactions between neighbors may occur. This bond is called molecular or van der Waals (as in a hydrogen molecule). But forces of electrostatic attraction can also arise between neutral atoms, then no rearrangements occur in the electronic shells of the atoms. Mutual repulsion as electron shells approach each other shifts the center of gravity of negative charges relative to positive ones. Each atom induces an electric dipole in the other, and this leads to their attraction. This is the action of intermolecular forces or van der Waals forces, which have a large radius of action.
Because a hydrogen atom is so small and its electron can be easily dislodged, it is often attracted to two atoms at once, forming a hydrogen bond. Hydrogen bonding is also responsible for the interaction of water molecules with each other. It explains many of the unique properties of water and ice (Fig. 7.4).
Covalent bond(or atomic) is achieved due to the internal interaction of neutral atoms. An example of such a bond is the bond in the methane molecule. The highly bonded variety of carbon is diamond (four hydrogen atoms are replaced by four carbon atoms).
So, carbon built on covalent bond, forms a diamond-shaped crystal. Each atom is surrounded by four atoms, forming a regular tetrahedron. But each of them is also the vertex of the neighboring tetrahedron. Under other conditions, the same carbon atoms crystallize into graphite. In graphite they are also connected by atomic bonds, but form planes of hexagonal honeycomb cells capable of shear. The distance between the atoms located at the vertices of the hexahedrons is 0.142 nm. The layers are located at a distance of 0.335 nm, i.e. are weakly bonded, so graphite is plastic and soft (Fig. 7.5). In 1990 there was a boom research work caused by a message about the receipt of a new substance - fullerite, consisting of carbon molecules - fullerenes. This form of carbon is molecular, i.e. The minimum element is not an atom, but a molecule. It is named after the architect R. Fuller, who in 1954 received a patent for building structures made of hexagons and pentagons that make up a hemisphere. Molecule from 60 carbon atoms with a diameter of 0.71 nm was discovered in 1985, then molecules were discovered, etc. They all had stable surfaces,
but the most stable molecules were C 60 and WITH 70 . It is logical to assume that graphite is used as a starting material for the synthesis of fullerenes. If this is so, then the radius of the hexagonal fragment should be 0.37 nm. But it turned out to be equal to 0.357 nm. This 2% difference is due to the fact that the carbon atoms are located on spherical surface at the vertices there are 20 regular hexahedrons, inherited from graphite, and 12 regular pentahedrons, i.e. The design resembles a soccer ball. It turns out that when “stitched” into a closed sphere, some of the flat hexahedrons turned into pentahedrons. At room temperature, C60 molecules condense into a structure where each molecule has 12 neighbors spaced 0.3 nm apart. At T= 349 K, a first-order phase transition occurs - the lattice is rearranged into a cubic one. The crystal itself is a semiconductor, but when an alkali metal is added to the C 60 crystalline film, superconductivity occurs at a temperature of 19 K. If one or another atom is introduced into this hollow molecule, it can be used as the basis for creating a storage medium with ultra-high information density: the recording density will reach 4-10 12 bits/cm 2 . For comparison, a film of ferromagnetic material gives a recording density of the order of 10 7 bits/cm 2, and optical disks, i.e. laser technology, - 10 8 bits/cm 2 . This carbon also has other unique properties, especially important in medicine and pharmacology.
Manifests itself in metal crystals metal connection, when all atoms in a metal give up their valence electrons “for collective use.” They are weakly bound to the atomic skeletons and can move freely along the crystal lattice. About 2/5 chemical elements are made up of metals. In metals (except mercury), a bond is formed when vacant orbitals of metal atoms overlap and electrons are removed due to the formation of a crystal lattice. It turns out that the lattice cations are enveloped in electron gas. A metallic bond occurs when atoms come together at a distance smaller than the size of the cloud of outer electrons. With this configuration (the Pauli principle), the energy of the outer electrons increases, and the neighboring nuclei begin to attract these outer electrons, blurring the electron clouds, evenly distributing them throughout the metal and turning them into an electron gas. This is how conduction electrons arise, which explain the high electrical conductivity of metals. In ionic and covalent crystals, the outer electrons are practically bound, and the conductivity of these solids is very small, they are called insulators.
The internal energy of liquids is determined by the sum of the internal energies of macroscopic subsystems into which it can be mentally divided, and the energies of interaction of these subsystems. The interaction is carried out through molecular forces with a radius of action of the order of 10 -9 m. For macrosystems, the interaction energy is proportional to the contact area, so it is small, like the fraction of the surface layer, but this is not necessary. It is called surface energy and should be taken into account in problems involving surface tension. Typically, liquids occupy a larger volume with equal weight, i.e., they have a lower density. But why do the volumes of ice and bismuth decrease during melting and, even after the melting point, maintain this trend for some time? It turns out that these substances are liquid state more dense.
In a liquid, each atom is acted upon by its neighbors, and it oscillates inside the anisotropic potential well that they create. Unlike a solid body, this hole is shallow, since distant neighbors have almost no influence. The immediate environment of particles in a liquid changes, i.e. the liquid flows. When a certain temperature is reached, the liquid will boil; during boiling, the temperature remains constant. The incoming energy is spent on breaking the bonds, and the liquid, when completely broken, turns into gas.
The densities of liquids are much greater than the densities of gases at the same pressures and temperatures. Thus, the volume of water at boiling is only 1/1600 of the volume of the same mass of water vapor. The volume of liquid depends little on pressure and temperature. Under normal conditions (20 °C and pressure 1.013 10 5 Pa), water occupies a volume of 1 liter. When the temperature drops to 10 °C, the volume decreases only by 0.0021, and when the pressure increases, the volume decreases by half.
Although there is no simple ideal model of a liquid yet, its microstructure has been sufficiently studied and makes it possible to qualitatively explain most of its macroscopic properties. The fact that in liquids the cohesion of molecules is weaker than in a solid body was noted by Galileo; He was surprised that large drops of water accumulated on cabbage leaves and did not spread over the leaf. Spilled mercury or drops of water on a greasy surface take the form of small balls due to adhesion. If molecules of one substance are attracted to molecules of another substance, we speak of wetting, for example glue and wood, oil and metal (despite the enormous pressure, the oil is retained in the bearings). But water rises in thin tubes called capillaries, and the thinner the tube, the higher it rises. There can be no other explanation other than the effect of wetting water and glass. The wetting forces between glass and water are greater than between water molecules. With mercury, the effect is the opposite: the wetting of mercury and glass is weaker than the adhesion forces between mercury atoms. Galileo noticed that a needle lubricated with fat could float on water, although this contradicted Archimedes' law. When the needle floats, you can
but notice a slight deflection of the surface of the water, trying to straighten out, as it were. The adhesion forces between water molecules are sufficient to prevent the needle from falling into the water. The surface layer protects water like a film, this is surface tension, which tends to give the shape of water the smallest surface - spherical. But the needle will no longer float on the surface of the alcohol, because when alcohol is added to the water, the surface tension decreases and the needle sinks. Soap also reduces surface tension, so hot soapy foam, penetrating into cracks and crevices, better washes away dirt, especially those containing grease, whereas clean water would simply curl into droplets.
Plasma is the fourth state of matter, which is a gas made up of a collection of charged particles interacting over long distances. In this case, the number of positive and negative charges is approximately equal, so that the plasma is electrically neutral. Of the four elements, plasma corresponds to fire. To transform a gas into a plasma state, it must be ionize, remove electrons from atoms. Ionization can be accomplished by heating, electrical discharge, or hard radiation. Matter in the Universe is mainly in an ionized state. In stars, ionization is caused thermally, in rarefied nebulae and interstellar gas - by ultraviolet radiation from stars. Our Sun also consists of plasma; its radiation ionizes the upper layers earth's atmosphere, called ionosphere, the possibility of long-distance radio communication depends on its condition. In terrestrial conditions, plasma is rarely found - in fluorescent lamps or in an electric welding arc. In laboratories and technology, plasma is most often obtained by electric discharge. In nature, lightning does this. During ionization by a discharge, electron avalanches occur, similar to a chain reaction process. To obtain thermonuclear energy, the injection method is used: gas ions accelerated to very high speeds are injected into magnetic traps, attracting electrons from environment, forming plasma. Pressure ionization - shock waves - is also used. This method of ionization occurs in super-dense stars and possibly in the Earth's core.
Any force acting on ions and electrons causes electricity. If it is not coupled to external fields and is not closed inside the plasma, it becomes polarized. Plasma obeys gas laws, but when a magnetic field is applied, which regulates the movement of charged particles, it exhibits properties that are completely unusual for a gas. In a strong magnetic field, particles begin to spin around field lines, and they move freely along the magnetic field. They say that this helical motion shifts the structure of the field lines and the field is “frozen” into the plasma. Rarefied plasma is described by a system of particles, while denser plasma is described by a liquid model.
The high electrical conductivity of plasma is its main difference from gas. The conductivity of the cold plasma of the solar surface (0.8 10 -19 J) reaches the conductivity of metals, and at thermonuclear temperature (1.6 10 -15 J) hydrogen plasma conducts current 20 times better than copper under normal conditions. Since plasma is capable of conducting current, the model of a conducting liquid is often applied to it. She is considered continuous medium, although compressibility distinguishes it from ordinary liquid, this difference appears only in flows whose speed more speed sound. The behavior of a conducting fluid is studied in a science called magnetic hydrodynamics. In space, any plasma is an ideal conductor, and the laws of the frozen field have wide application. The model of a conducting liquid allows us to understand the mechanism of plasma confinement by a magnetic field. Thus, plasma streams are emitted from the Sun, affecting the Earth’s atmosphere. The flow itself does not have a magnetic field, but an extraneous field cannot penetrate into it according to the law of freezing. Plasma solar streams push extraneous interplanetary magnetic fields out of the vicinity of the Sun. A magnetic cavity appears where the field is weaker. When these corpuscular plasma flows approach the Earth, they collide with the Earth's magnetic field and are forced to flow around it according to the same law. It turns out to be a kind of cavity where the magnetic field is collected and where plasma flows do not penetrate. Charged particles that were detected by rockets and satellites accumulate on its surface - this is the Earth's outer radiation belt. These ideas were also used in solving problems of plasma confinement by a magnetic field in special devices - tokamaks (from the abbreviation of the words: toroidal chamber, magnet). With fully ionized plasma contained in these and other systems, hopes are pinned on obtaining a controlled thermonuclear reaction on Earth. This would provide a clean and cheap source of energy ( sea water). Work is also underway to produce and retain plasma using focused laser radiation.
Presentation on the topic "Alcohols" in chemistry in powerpoint format. The presentation for schoolchildren contains 12 slides, which, from a chemical point of view, talk about alcohols, their physical properties, and reactions with hydrogen halides.
Fragments from the presentation
From the history
Did you know that back in the 4th century. BC e. did people know how to make drinks containing ethyl alcohol? Wine was produced by fermenting fruit and berry juices. However, they learned to extract the intoxicating component from it much later. In the 11th century alchemists detected vapors of a volatile substance that was released when wine was heated.
Physical properties
- Lower alcohols are liquids that are highly soluble in water, colorless, and odorless.
- Higher alcohols are solid substances that are insoluble in water.
Feature of physical properties: state of aggregation
- Methyl alcohol (the first representative of the homologous series of alcohols) is a liquid. Maybe it has a high molecular weight? No. Much less than carbon dioxide. Then what is it?
- It turns out that the whole point is in the hydrogen bonds that form between alcohol molecules and prevent individual molecules from flying away.
Feature of physical properties: solubility in water
- Lower alcohols are soluble in water, higher alcohols are insoluble. Why?
- Hydrogen bonds are too weak to hold the alcohol molecule, which has a large insoluble portion, between water molecules.
Feature of physical properties: contraction
- Why do people never use volume, but only mass, when solving calculation problems?
- Mix 500 ml of alcohol and 500 ml of water. We get 930 ml of solution. The hydrogen bonds between the molecules of alcohol and water are so strong that the total volume of the solution decreases, its “compression” (from the Latin contraktio - compression).
Are alcohols acids?
- Alcohols react with alkali metals. In this case, the hydrogen atom of the hydroxyl group is replaced by a metal. Looks like acid.
- But the acidic properties of alcohols are too weak, so weak that alcohols do not affect indicators.
Friendship with the traffic police.
- Are alcohols friendly with traffic police? But how!
- Have you ever been stopped by a traffic police inspector? Have you ever breathed into a tube?
- If you are unlucky, the alcohol undergoes an oxidation reaction, causing the color to change and you have to pay a fine.
We give water 1
Removal of water - dehydration can be intramolecular if the temperature is more than 140 degrees. This requires a catalyst - concentrated sulfuric acid.
Give back water 2
If the temperature is reduced and the catalyst remains the same, then intermolecular dehydration will occur.
Reaction with hydrogen halides.
This reaction is reversible and requires a catalyst - concentrated sulfuric acid.
To be friends or not to be friends with alcohol.
Interesting question. Alcohol is a xenobiotic - substances not found in the human body, but affecting its vital functions. It all depends on the dose.
- Alcohol is a nutrient that provides the body with energy. In the Middle Ages, the body received about 25% of its energy through alcohol consumption.
- Alcohol is a medicine that has a disinfectant and antibacterial effect.
- Alcohol is a poison that disrupts natural biological processes, destroys internal organs and the psyche, and leads to death if consumed in excess.
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0