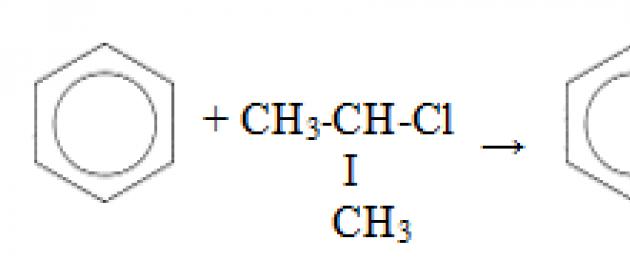DEFINITION
Benzene(cyclohexatriene - 1,3,5) - organic matter, the simplest representative of a number of aromatic hydrocarbons.
Formula - C 6 H 6 (structural formula - Fig. 1). Molecular mass – 78, 11.
Rice. 1. Structural and spatial formulas of benzene.
All six carbon atoms in the benzene molecule are in the sp 2 hybrid state. Each carbon atom forms 3σ bonds with two other carbon atoms and one hydrogen atom lying in the same plane. Six carbon atoms form a regular hexagon (σ-skeleton of the benzene molecule). Each carbon atom has one unhybridized p-orbital, which contains one electron. Six p-electrons form a single π-electron cloud (aromatic system), which is depicted as a circle inside a six-membered cycle. The hydrocarbon radical derived from benzene is called C 6 H 5 - - phenyl (Ph-).
Chemical properties of benzene
Benzene is characterized by substitution reactions proceeding according to the electrophilic mechanism:
- halogenation (benzene interacts with chlorine and bromine in the presence of catalysts - anhydrous AlCl 3, FeCl 3, AlBr 3)
C 6 H 6 + Cl 2 \u003d C 6 H 5 -Cl + HCl;
- nitration (benzene easily reacts with a nitrating mixture - a mixture of concentrated nitric and sulfuric acids)

- alkylation with alkenes
C 6 H 6 + CH 2 \u003d CH-CH 3 → C 6 H 5 -CH (CH 3) 2;
Addition reactions to benzene lead to the destruction of the aromatic system and proceed only under harsh conditions:
- hydrogenation (the reaction proceeds when heated, the catalyst is Pt)

- addition of chlorine (occurs under the action of UV radiation with the formation of a solid product - hexachlorocyclohexane (hexachlorane) - C 6 H 6 Cl 6)

Like any organic compound, benzene enters into a combustion reaction to form as reaction products carbon dioxide and water (burns with a smoky flame):
2C 6 H 6 + 15O 2 → 12CO 2 + 6H 2 O.
Physical properties of benzene
Benzene is a colorless liquid, but has a specific pungent odor. Forms an azeotropic mixture with water, mixes well with ethers, gasoline and various organic solvents. Boiling point - 80.1C, melting point - 5.5C. Toxic, carcinogen (i.e. contributes to the development of cancer).
Obtaining and using benzene
The main methods for obtaining benzene:
— dehydrocyclization of hexane (catalysts - Pt, Cr 3 O 2)
CH 3 -(CH 2) 4 -CH 3 → C 6 H 6 + 4H 2;
- dehydrogenation of cyclohexane (the reaction proceeds when heated, the catalyst is Pt)
C 6 H 12 → C 6 H 6 + 4H 2;
– trimerization of acetylene (the reaction proceeds when heated to 600C, the catalyst is activated carbon)
3HC≡CH → C 6 H 6 .
Benzene serves as a raw material for the production of homologues (ethylbenzene, cumene), cyclohexane, nitrobenzene, chlorobenzene, and other substances. Previously, benzene was used as an additive to gasoline to increase its octane number, however, now, due to its high toxicity, the content of benzene in fuel is strictly regulated. Sometimes benzene is used as a solvent.
Examples of problem solving
EXAMPLE 1
| Exercise | Write down the equations with which you can carry out the following transformations: CH 4 → C 2 H 2 → C 6 H 6 → C 6 H 5 Cl. |
| Solution | To obtain acetylene from methane, the following reaction is used: 2CH 4 → C 2 H 2 + 3H 2 (t = 1400C). Obtaining benzene from acetylene is possible by the reaction of trimerization of acetylene, which occurs when heated (t = 600C) and in the presence of activated carbon: 3C 2 H 2 → C 6 H 6 . The chlorination reaction of benzene to obtain chlorobenzene as a product is carried out in the presence of iron (III) chloride: C 6 H 6 + Cl 2 → C 6 H 5 Cl + HCl. |
EXAMPLE 2
| Exercise | To 39 g of benzene in the presence of iron (III) chloride was added 1 mol of bromine water. What amount of the substance and how many grams of what products did this result in? |
| Solution | Let us write the equation for the reaction of benzene bromination in the presence of iron (III) chloride: C 6 H 6 + Br 2 → C 6 H 5 Br + HBr. The reaction products are bromobenzene and hydrogen bromide. Molar mass of benzene, calculated using the table chemical elements DI. Mendeleev - 78 g/mol. Find the amount of benzene substance: n(C 6 H 6) = m(C 6 H 6) / M(C 6 H 6); n(C 6 H 6) = 39/78 = 0.5 mol. According to the condition of the problem, benzene reacted with 1 mol of bromine. Consequently, benzene is in short supply and further calculations will be made for benzene. According to the reaction equation n (C 6 H 6): n (C 6 H 5 Br) : n (HBr) \u003d 1: 1: 1, therefore n (C 6 H 6) \u003d n (C 6 H 5 Br) \u003d: n(HBr) = 0.5 mol. Then, the masses of bromobenzene and hydrogen bromide will be equal: m(C 6 H 5 Br) = n(C 6 H 5 Br)×M(C 6 H 5 Br); m(HBr) = n(HBr)×M(HBr). Molar masses of bromobenzene and hydrogen bromide, calculated using the table of chemical elements of D.I. Mendeleev - 157 and 81 g/mol, respectively. m(C 6 H 5 Br) = 0.5×157 = 78.5 g; m(HBr) = 0.5 x 81 = 40.5 g. |
| Answer | The reaction products are bromobenzene and hydrogen bromide. The masses of bromobenzene and hydrogen bromide are 78.5 and 40.5 g, respectively. |
Arenes are aromatic hydrocarbons containing one or more benzene rings. The benzene ring is made up of 6 carbon atoms, between which double and single bonds alternate.
It is important to note that the double bonds in the benzene molecule are not fixed, but constantly move in a circle.
Arenes are also called aromatic hydrocarbons. First member homologous series- benzene - C 6 H 6 . The general formula for their homologous series is C n H 2n-6.
For a long time, the structural formula of benzene remained a mystery. The formula proposed by Kekule with two triple bonds could not explain the fact that benzene does not enter into addition reactions. As mentioned above, according to modern concepts, double bonds in a molecule are constantly moving, so it is more correct to draw them in the form of a ring.
Double bonds form a conjugation in the benzene molecule. All carbon atoms are in a state of sp 2 hybridization. Valence angle - 120°.
Nomenclature and isomerism of arenes
The names of arenes are formed by adding the names of substituents to the main chain - the benzene ring: benzene, methylbenzene (toluene), ethylbenzene, propylbenzene, etc. Substituents are, as usual, listed in alphabetical order. If there are several substituents in the benzene ring, then the shortest path between them is chosen.

Arenes are characterized by structural isomerism associated with the position of substituents. For example, two substituents on a benzene ring may be in different positions.
The name of the position of the substituents in the benzene ring is formed on the basis of their location relative to each other. It is denoted by the prefixes ortho-, meta- and para. Below you will find mnemonic hints for their successful memorization;)

Getting arenas
Arenas are obtained in several ways:

Chemical properties of arenes
Arenes are aromatic hydrocarbons that contain a benzene ring with conjugated double bonds. This feature makes addition reactions difficult (but still possible!)
Remember that, unlike other unsaturated compounds, benzene and its homologues do not discolor bromine water and potassium permanganate solution.

© Bellevich Yury Sergeevich 2018-2020
This article was written by Yury Sergeevich Bellevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without the prior consent of the copyright holder is punishable by law. To obtain the materials of the article and permission to use them, please contact
Aromatic HCs (arenas) are hydrocarbons whose molecules contain one or more benzene rings.
Examples of aromatic hydrocarbons:

Benzene row arenas (monocyclic arenas)
General formula:C n H 2n-6 , n≥6
The simplest representative of aromatic hydrocarbons is benzene, its empirical formula is C 6 H 6 .
The electronic structure of the benzene molecule
The general formula of C n H 2 n -6 monocyclic arenes shows that they are unsaturated compounds.
In 1856, the German chemist A.F. Kekule proposed a cyclic formula for benzene with conjugated bonds (single and double bonds alternate) - cyclohexatriene-1,3,5: 
This structure of the benzene molecule did not explain many of the properties of benzene:
- for benzene, substitution reactions are characteristic, and not addition reactions characteristic of unsaturated compounds. Addition reactions are possible, but they are more difficult than for;
- benzene does not enter into reactions that are qualitative reactions to unsaturated hydrocarbons (with bromine water and KMnO 4 solution).
Electron diffraction studies carried out later showed that all bonds between carbon atoms in a benzene molecule have the same length of 0.140 nm (the average value between the length of a simple C-C connections 0.154 nm and C=C double bond 0.134 nm). The angle between the bonds at each carbon atom is 120°. The molecule is a regular flat hexagon.
Modern theory to explain the structure of the C 6 H 6 molecule uses the concept of hybridization of atomic orbitals.
The carbon atoms in benzene are in a state of sp 2 hybridization. Each "C" atom forms three σ-bonds (two with carbon atoms and one with a hydrogen atom). All σ-bonds are in the same plane:
Each carbon atom has one p-electron, which does not participate in hybridization. The unhybridized p-orbitals of carbon atoms are in a plane perpendicular to the plane of σ-bonds. Each p-cloud overlaps with two neighboring p-clouds, and as a result, a single conjugated π-system is formed (remember the effect of conjugation of p-electrons in the 1,3-butadiene molecule, discussed in the topic “Diene hydrocarbons”):
The combination of six σ-bonds with a single π-system is called aromatic bond.
A ring of six carbon atoms linked by an aromatic bond is called benzene ring, or benzene nucleus.
In accordance with modern ideas about the electronic structure of benzene, the C 6 H 6 molecule is depicted as follows:

Physical properties of benzene
Benzene under normal conditions is a colorless liquid; t o pl = 5.5 o C; t o kip. = 80 about C; has a characteristic smell; immiscible with water, good solvent, highly toxic.
Chemical properties of benzene
The aromatic bond determines the chemical properties of benzene and other aromatic hydrocarbons.
The 6π-electron system is more stable than conventional two-electron π-bonds. Therefore, addition reactions are less typical for aromatic hydrocarbons than for unsaturated hydrocarbons. The most typical for arenes are substitution reactions.
I. Substitution reactions
1.Halogenation

2. Nitration
The reaction is carried out with a mixture of and acids (nitrating mixture): 
3. Sulfonation

4. Alkylation (replacement of the "H" atom by an alkyl group) - Friedel-Crafts reactions, homologues of benzene are formed:

Instead of haloalkanes, alkenes can be used (in the presence of a catalyst - AlCl 3 or inorganic acid):

II. Addition reactions
1. Hydrogenation

2. Addition of chlorine

III.Oxidation reactions
1. Combustion
2C 6 H 6 + 15O 2 → 12CO 2 + 6H 2 O
2. Not complete oxidation (KMnO 4 or K 2 Cr 2 O 7 in an acidic environment). The benzene ring is resistant to oxidizing agents. The reaction does not occur.
Getting benzene
In industry:
1) oil and coal processing;
2) dehydrogenation of cyclohexane:

3) dehydrocyclization (aromatization) of hexane:

In the laboratory:
Fusion of salts of benzoic acid with:

Isomerism and nomenclature of benzene homologues
Any benzene homologue has a side chain, i.e. alkyl radicals attached to the benzene ring. The first homologue of benzene is a benzene nucleus linked to a methyl radical: 
Toluene has no isomers, since all positions in the benzene ring are equivalent.
For subsequent homologues of benzene, one type of isomerism is possible - side chain isomerism, which can be of two types:
1) isomerism of the number and structure of substituents;
2) isomerism of the position of substituents.



Physical properties of toluene
Toluene- a colorless liquid with a characteristic odor, insoluble in water, soluble in organic solvents. Toluene is less toxic than benzene.
Chemical properties of toluene
I. Substitution reactions
1. Reactions involving the benzene ring
Methylbenzene enters into all substitution reactions in which benzene is involved, and at the same time exhibits a higher reactivity, the reactions proceed at a faster rate.
The methyl radical contained in the toluene molecule is a substituent of the genus, therefore, as a result of substitution reactions in the benzene nucleus, ortho- and para-derivatives of toluene are obtained or, with an excess of the reagent, tri-derivatives of the general formula:

a) halogenation


With further chlorination, dichloromethylbenzene and trichloromethylbenzene can be obtained:

II. Addition reactions
hydrogenation

III.Oxidation reactions
1. Combustion
C 6 H 5 CH 3 + 9O 2 → 7CO 2 + 4H 2 O
2. Incomplete oxidation
Unlike benzene, its homologues are oxidized by some oxidizing agents; in this case, the side chain undergoes oxidation, in the case of toluene, the methyl group. Mild oxidizing agents like MnO 2 oxidize it to an aldehyde group, stronger oxidizing agents (KMnO 4) cause further oxidation to an acid: 
Any homologue of benzene with one side chain is oxidized by a strong oxidizing agent such as KMnO4 to benzoic acid, i.e. there is a break in the side chain with the oxidation of its cleaved off part to CO 2; For example: 
In the presence of several side chains, each of them is oxidized to a carboxyl group and as a result polybasic acids are formed, for example:

Getting toluene:
In industry:
1) oil and coal processing;
2) dehydrogenation of methylcyclohexane:

3) dehydrocyclization of heptane:
In the laboratory:
1) Friedel-Crafts alkylation;
2) Wurtz-Fittig reaction(reaction of sodium with a mixture of halobenzene and haloalkane).
The first group of reactions is substitution reactions. We said that arenes do not have multiple bonds in the molecular structure, but contain a conjugated system of six electrons, which is very stable and gives additional strength to the benzene ring. Therefore, in chemical reactions, first of all, the substitution of hydrogen atoms occurs, and not the destruction of the benzene ring.
We have already encountered substitution reactions when talking about alkanes, but for them these reactions proceeded according to a radical mechanism, and for arenes the ionic mechanism of substitution reactions is characteristic.
First chemical property - halogenation. Substitution of a hydrogen atom for a halogen atom - chlorine or bromine.
The reaction proceeds when heated and always with the participation of a catalyst. In the case of chlorine, it can be aluminum chloride or iron chloride three. The catalyst polarizes the halogen molecule, resulting in heterolytic bond breaking and ions are obtained.
The positively charged chloride ion reacts with benzene.
If the reaction occurs with bromine, then iron tribromide or aluminum bromide acts as a catalyst.

It is important to note that the reaction occurs with molecular bromine and not with bromine water. Benzene does not react with bromine water.
The halogenation of benzene homologues has its own characteristics. In the toluene molecule, the methyl group facilitates substitution in the ring, the reactivity increases, and the reaction proceeds under milder conditions, that is, already at room temperature.

It is important to note that the substitution always occurs in the ortho and para positions, so a mixture of isomers is obtained.
Second property - nitration of benzene, the introduction of a nitro group into the benzene ring.
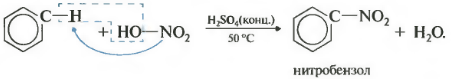
A heavy yellowish liquid with the smell of bitter almonds is formed - nitrobenzene, so the reaction can be qualitative for benzene. For nitration, a nitrating mixture of concentrated nitric and sulfuric acids is used. The reaction is carried out by heating.
Let me remind you that for the nitration of alkanes in the Konovalov reaction, dilute nitric acid was used without the addition of sulfuric acid.
In the nitration of toluene, as well as in the halogenation, a mixture of ortho- and para-isomers is formed.

Third property - alkylation of benzene with haloalkanes.

This reaction allows the introduction of a hydrocarbon radical into the benzene ring and can be considered a method for obtaining benzene homologues. Aluminum chloride is used as a catalyst, which promotes the decomposition of the haloalkane molecule into ions. It also needs heating.
Fourth property - alkylation of benzene with alkenes.

In this way, for example, cumene or ethylbenzene can be obtained. The catalyst is aluminum chloride.
2. Reactions of addition to benzene
The second group of reactions is addition reactions. We said that these reactions are not characteristic, but they are possible under rather harsh conditions with the destruction of the pi-electron cloud and the formation of six sigma bonds.
Fifth property in the general list - hydrogenation, addition of hydrogen.
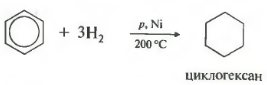
Temperature, pressure, catalyst nickel or platinum. Toluene is able to react in the same way.
sixth property - chlorination. Please note that we are talking specifically about the interaction with chlorine, since bromine does not enter into this reaction.
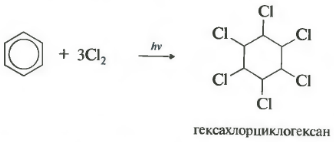
The reaction proceeds under hard ultraviolet irradiation. Hexachlorocyclohexane, another name for hexachlorane, is formed, a solid.
It is important to remember that for benzene not possible addition reactions of hydrogen halides (hydrohalogenation) and addition of water (hydration).
3. Substitution in the side chain of benzene homologues
The third group of reactions concerns only benzene homologues - this is a substitution in the side chain.
seventh a property in the general list is halogenation at the alpha carbon atom in the side chain.
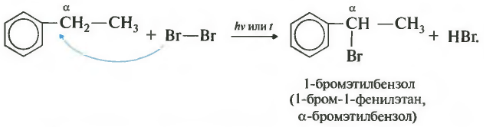
The reaction occurs when heated or irradiated, and always only at the alpha carbon. As the halogenation continues, the second halogen atom will return to the alpha position.
4. Oxidation of benzene homologues
The fourth group of reactions is oxidation.
The benzene ring is too strong, so benzene does not oxidize potassium permanganate - does not discolor its solution. This is very important to remember.
On the other hand, benzene homologues are oxidized with an acidified solution of potassium permanganate when heated. And this is the eighth chemical property.
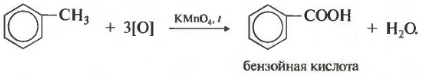
It turns out benzoic acid. Discoloration of the solution is observed. In this case, no matter how long the carbon chain of the substituent is, it always breaks after the first carbon atom and the alpha atom is oxidized to a carboxyl group with the formation of benzoic acid. The rest of the molecule is oxidized to the corresponding acid or, if it is only one carbon atom, to carbon dioxide.
If the benzene homologue has more than one hydrocarbon substituent on the aromatic ring, then the oxidation occurs according to the same rules - the carbon in the alpha position is oxidized.
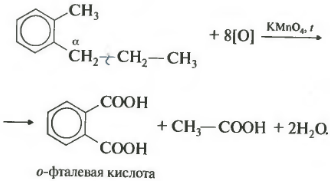
In this example, a dibasic aromatic acid is obtained, which is called phthalic acid.
In a special way, I note the oxidation of cumene, isopropylbenzene, with atmospheric oxygen in the presence of sulfuric acid.

This is the so-called cumene method for producing phenol. As a rule, one has to deal with this reaction in matters relating to the production of phenol. This is the industrial way.
ninth property - combustion, complete oxidation with oxygen. Benzene and its homologues burn to carbon dioxide and water.
Let us write the equation for the combustion of benzene in a general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because, after all, in chemical reactions, atoms do not go anywhere, but the order of bonds between them simply changes. So there will be as many carbon dioxide molecules as there are carbon atoms in an arene molecule, since the molecule contains one carbon atom. That is n CO 2 molecules. There will be half as many water molecules as hydrogen atoms, that is, (2n-6) / 2, which means n-3.
There are the same number of oxygen atoms on the left and on the right. On the right, there are 2n from carbon dioxide, because there are two oxygen atoms in each molecule, plus n-3 from water, for a total of 3n-3. On the left, there are the same number of oxygen atoms - 3n-3, which means there are half as many molecules, because the molecule contains two atoms. That is (3n-3)/2 oxygen molecules.
Thus, we have compiled the equation for the combustion of benzene homologues in a general form.
PRTSVSH (F) FGBOU VPO
Department of "Fire Safety"
Test
in the discipline "Theory of combustion and explosions"
Task number 1
Determine the specific theoretical quantities and volume of air required for the complete combustion of benzene vapor. The conditions in which the air is located are characterized by temperature Tv and pressure Pv, and benzene vapor - temperature Tg and pressure Pg. Express the calculation results in the following units: ; ;;;
Initial data (N - group number, n - number according to the list of students:
TV=300+(-1) N *2*N-(-1) n *0.2*n= 277.6 K
Pv \u003d? 10 3 \u003d 95900 Pa;
Тg=300?(?1) N?2?N?(?1) n?0.2?n= 321.6 K;
Pr \u003d? 10 3 \u003d 79400 Pa.
С6Н6+7.5О2+7.5?3.76N2=6CO2+3pO+7.5?3.76N2+Qp (1),
where Qp - heat chemical reaction. From this equation, it is possible to determine the stoichiometric coefficients of benzene and molecular oxygen: Vg = 1, V0 = 7.5
2. Specific theoretical amount of air - the number of kilomoles of air that are necessary for the complete combustion of one kilomol of benzene is calculated by the formula:
where 4.76 is the amount of air that contains a unit of oxygen, \u003d is the ratio of the stoichiometric coefficients of molecular oxygen (Vo) and benzene (Vg)
Substituting in (d) the values of Vo and Vg, we obtain:
3. The volume of air required for the complete combustion of one kilomole of benzene is determined as follows:
where is the volume of one kilomole of air at temperature Tv and pressure Pv. The value is calculated using the formula
where 22.4 is the molar volume of gas under normal conditions, Po = 101325 Pa is normal pressure, To = 273 K is normal temperature.
Substituting Tv, To, Pv, Po in (5), we obtain
The specific theoretical air volume is calculated by the formula (4):
4. The volume of air required for the complete combustion of a unit volume of gaseous fuel is determined as follows:
where is the volume of one kilomole of fuel - benzene vapor at temperature Tg and pressure Pg. Given that
and substituting (8) and (5) into (7), we obtain the following expression for the specific theoretical air volume:
We calculate the value of this parameter of the combustion process:
The volume of air required for the complete combustion of one kilogram of benzene is determined as follows:
where - the molar mass of fuel is the mass of one kilomole of benzene, expressed in kilograms. The molar mass of benzene is numerically equal to its molecular weight is found by the formula:
Ac?nc + An?nn, UiAi?ni (11)
where Ac and An are the atomic weights of carbon and hydrogen, nc and nn are the numbers of carbon atoms in the benzene molecule. Substituting the values Ac = 12, nc = 6, An = 1, nn = 6, we get:
We find the specific theoretical volume of air by substituting the values of n into and into formula (10):
Calculation result:
Task number 2
Determine the specific theoretical quantity, volume and composition of benzene combustion products, if the coefficient of excess air c, temperature Tp and pressure Pp of combustion products, temperature Tg and pressure Pg of benzene vapor are known. Express the calculation results in mole fractions (in percent) and in the following units: ; ;;
Initial data:
c=1.5+(?1) N?0.1?N?(?1) n?0.01?n = 0.2;
Rp \u003d? 10 3 \u003d 68400 Pa;
Tp=1600?(?1) N?20?N?(?1) n?2?n = 1816 K;
Тg=273?(?1) N?2?N+(?1) n?0.2?n = 295.4 K;
Rg \u003d? 10 3 \u003d 111600 Pa;
solution (N=11, n=2).
1. We write the stoichiometric equation for the reaction of benzene combustion in air:
C 6 H 6 +7.5O 2 +7.5? 3.76N 2 \u003d 6CO 2 + 3H 2 O + 7.5? 3.76N 2 + Qp, (1)
where Qp is the heat of a chemical reaction. From this equation, we determine the following stoichiometric coefficients:
V CO2 \u003d 6, V pO \u003d 3, V C6H6 \u003d 1, V O2 \u003d 7.5, V N2 \u003d 7.5? 3.76
2. Determine the estimated amount of combustion products of one kilomole of fuel:
Substituting in (2) the values of the stoichiometric coefficients of combustion products and fuel, we obtain:
3. Specific theoretical amount of air - the number of kilomoles of air necessary for the complete combustion of one kilomol of fuel, we determine using the formula:
Where 4.76 is the amount of air that contains a unit of oxygen,
Ratio of stoichiometric coefficients of molecular oxygen and benzene.
Substituting in (4) the values V O2 =7.5 and V C6H6 =1 , we obtain:
4. The excess amount of air that falls on 1 Kmol of fuel is determined by the expression:
benzene steam combustion air
Substituting in this expression the values
37,7(0,2-1)=30,16(7)
5. The total amount of combustion products per unit amount of fuel substance is determined by the sum:
After substituting the values and we get:
6. Mole fractions of combustion products, expressed as a percentage, are determined as follows:
In formulas (9) for the mole fractions of nitrogen and oxygen in the combustion products, 0.79 and 0.21 are the mole fractions of these substances in the air, the excess of which leads to an increase in the proportion of nitrogen and the appearance of oxygen in the combustion products.
7. To determine the specific volumes and products of combustion, it is necessary to calculate their molar volume - the volume of one kilomole of gas under the conditions in which the products are located:
where 22.4 is the volume of one kilomole of gas under normal conditions, T 0 \u003d 273K - normal temperature, Po \u003d 101325 Pa - normal pressure.
Substituting in (10) the values, Po, To, we get:
The volume of products that are formed during the combustion of one kilogram of fuel, excluding excess air, is calculated as follows:
where - the molar mass of fuel is the mass of one kilomole of benzene, expressed in kilograms. The molar mass of benzene is found by the formula:
where Ac and An are the atomic weights of carbon (12) and hydrogen (1), n c and n n are the numbers of carbon (6) and hydrogen (6) atoms in benzene molecules (C 6 H 6).
Substituting the values, and in (12) we obtain
The excess volume of air per 1 kilogram of fuel is determined as follows:
where is the volume of one kilomole of excess air, which is part of the combustion products. Since the temperature and pressure of excess air correspond to the temperature and pressure of the combustion products, then \u003d \u003d 220.7.
Substituting this value, as well as in (14), we obtain:
To calculate the specific volume of products of complete combustion of fuel, we assume that benzene vapor has a temperature Tg at pressure:
where is the volume of one kilomole of benzene vapor at temperature Tg and pressure Pg. The molar volume of fuel is calculated by the formula:
Substituting the obtained value, and such values in (17), we obtain:
The excess volume of air per cubic meter of benzene vapor is determined as follows:
Substitution in (20) values \u003d 30.16 , \u003d and
gives the following result:
The total specific volume of combustion products, taking into account excess air, is determined by the sum
Calculation result:
X CO2 \u003d%; X H2O \u003d 4.4%; X N2 =%; X O2 \u003d 11.7%
Similar Documents
Calculation of the combustibility coefficient of nitrobenzene C6H5NO2 and carbon disulfide CS2. Equation for the combustion reaction of propyl acetate in air. Calculation of the volume of air and combustion products during the combustion of combustible gas. Determination of the flash point of toluene according to the formula of V. Blinov.
test, added 04/08/2017
Calculation of the volume of air and combustion products formed during the combustion of a substance. The equation for the combustion reaction of ethylene glycol in air. Combustion of a mixture of combustible gases. Calculation of the adiabatic combustion temperature for a stoichiometric mixture. combustion of propanol.
test, added 10/17/2012
Type of combustion and its main parameters. Chemical conversion of fuel and oxidant into combustion products. Equations of material and thermal balance of the combustion reaction. Influence of excess air coefficient on the composition of combustion products and combustion temperature.
test, added 01/17/2013
Determination of the volume of air required for the complete combustion of a unit mass of a combustible substance. The composition of the products of combustion of a unit mass of a combustible substance. Limits of flame propagation of gas, steam, dust-air mixtures. Explosive decomposition pressure.
term paper, added 12/23/2013
Development of measures to prevent the occurrence of fires and explosions, assessment of the conditions for their development and suppression. The concept of burnout rate, the method of its definition. The procedure for compiling the combustion reaction equation. Calculation of the volume of air required for ignition.
term paper, added 07/10/2014
Determination of the composition of products of complete combustion of gas. Calculation of the adiabatic combustion temperature of a gas mixture at constant volume and constant pressure. Kinetic reaction constants of self-ignition of natural gas. Limit of ignition of the gas mixture.
term paper, added 02/19/2014
Characterization of industrial methods for the alkylation of benzene with propylene. Principles of alkylation of benzene with olefins in chemical technology. Problems of designing technological installations for benzene alkylation. Description of the technology of the production process.
thesis, added 11/15/2010
Combustion is a powerful oxidation process. Types of combustion: smoldering and burning with a flame. Explosion like special case burning. Electrical properties of the flame. Variety of combustion products as a result of incomplete combustion of fuel. Filtration of smoke through water.
scientific work, added 07/29/2009
Determination of the volume of air required for the complete combustion of a given amount of propane. Calculation of the change in enthalpy, entropy and Gibbs energy, using the consequences of Hess's law. Determination of the molar mass equivalents of the oxidizing agent and reducing agent.
test, added 02/08/2012
Methods for determining the consumption of absorbent oil, the concentration of benzene in the absorbent oil leaving the absorber. Calculation of the diameter and height of the packed absorber. Determination of the required heating surface in the cube of the column and the consumption of heating steam.
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0