The variety of individual units (force, for example, could be expressed in kg, pounds, etc.) and systems of units created great difficulties in the worldwide exchange of scientific and economic achievements. Therefore, back in the 19th century, there was a need to create a unified international system that would include units of measurement of quantities used in all branches of physics. However, agreement to introduce such a system was adopted only in 1960.
International system of units is a correctly constructed and interconnected set physical quantities. It was adopted in October 1960 at the 11th General Conference on Weights and Measures. The abbreviated name of the system is SI. In Russian transcription - SI. (international system).
In the USSR, GOST 9867-61 was introduced in 1961, which established the preferable use of this system in all areas of science, technology, and teaching. Currently, the current GOST 8.417-81 “GSI. Units of physical quantities". This standard establishes the units of physical quantities used in the USSR, their names, designations and rules of application. It is developed in full accordance with the SI system and ST SEV 1052-78.
The C system consists of seven basic units, two additional units and a number of derivatives. In addition to SI units, the use of submultiples and multiples is allowed, obtained by multiplying the original values by 10 n, where n = 18, 15, 12, ... -12, -15, -18. The names of multiple and submultiple units are formed by adding the corresponding decimal prefixes:
exa (E) = 10 18; peta (P) = 10 15 ; tera (T) = 10 12 ; giga (G) = 10 9 ; mega (M) = 10 6 ;
miles (m) = 10 –3 ; micro (μ) = 10 –6; nano(n) = 10 –9; pico(p) = 10 –12;
femto (f) = 10 –15; atto(a) = 10 –18;
GOST 8.417-81 allows the use, in addition to the specified units, of a number of non-systemic units, as well as units temporarily permitted for use until the relevant international decisions are adopted.
The first group includes: ton, day, hour, minute, year, liter, light year, volt-ampere.
The second group includes: nautical mile, carat, knot, rpm.
1.4.4 Basic units of SI.
Unit of length – meter (m)
A meter is equal to 1650763.73 wavelengths in vacuum of radiation corresponding to the transition between the 2p 10 and 5d 5 levels of the krypton-86 atom.
The International Bureau of Weights and Measures and large national metrology laboratories have created installations for reproducing the meter in light wavelengths.
The unit of mass is kilogram (kg).
Mass is a measure of the inertia of bodies and their gravitational properties. A kilogram is equal to the mass of the international prototype of the kilogram.
The state primary standard of the SI kilogram is intended for reproduction, storage and transfer of the unit of mass to working standards.
The standard includes:
A copy of the international prototype of the kilogram - platinum-iridium prototype No. 12, which is a weight in the form of a cylinder with a diameter and height of 39 mm.
Equal-arm prismatic scales No. 1 for 1 kg with remote control from Ruphert (1895) and No. 2 manufactured at VNIIM in 1966.
Once every 10 years, the state standard is compared with a copy standard. Over 90 years, the mass of the state standard has increased by 0.02 mg due to dust, adsorption and corrosion.
Now mass is the only unit quantity that is determined through a real standard. This definition has a number of disadvantages - change in the mass of the standard over time, irreproducibility of the standard. Research is underway to express a unit of mass through natural constants, for example through the mass of a proton. It is also planned to develop a standard using a certain number of Si-28 silicon atoms. To solve this problem, first of all, the accuracy of measuring Avogadro's number must be increased.
The unit of time is second (s).
Time is one of the central concepts of our worldview, one of the most important factors in the life and activities of people. It is measured using stable periodic processes - the annual rotation of the Earth around the Sun, daily - the rotation of the Earth around its axis, and various oscillatory processes. The definition of the unit of time, the second, has changed several times in accordance with the development of science and the requirements for measurement accuracy. The current definition is:
A second is equal to 9192631770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium 133 atom.
Currently, a beam standard of time, frequency and length has been created, used by the time and frequency service. Radio signals allow the transmission of a unit of time, so it is widely available. The standard second error is 1·10 -19 s.
The unit of electric current is ampere (A)
An ampere is equal to the strength of an unchanging current, which, when passing through two parallel and straight conductors of infinite length and negligibly small cross-sectional area, located in a vacuum at a distance of 1 meter from each other, would cause on each section of the conductor 1 meter long an interaction force equal to 2 ·10 -7 N.
The error of the ampere standard is 4·10 -6 A. This unit is reproduced using the so-called current scales, which are accepted as the ampere standard. It is planned to use 1 volt as the main unit, since its reproduction error is 5·10 -8 V.
Unit of thermodynamic temperature – Kelvin (K)
Temperature is a value that characterizes the degree of heating of a body.
Since the invention of the Thermometer by Galileo, temperature measurement has been based on the use of one or another thermometric substance that changes its volume or pressure with a change in temperature.
All known temperature scales (Fahrenheit, Celsius, Kelvin) are based on some reference points to which different numerical values are assigned.
Kelvin and, independently of him, Mendeleev expressed considerations about the advisability of constructing a temperature scale based on one reference point, which was taken as the “triple point of water,” which is the equilibrium point of water in the solid, liquid and gaseous phases. It can currently be reproduced in special vessels with an error of no more than 0.0001 degrees Celsius. The lower limit of the temperature range is the absolute zero point. If this interval is divided into 273.16 parts, you get a unit of measurement called Kelvin.
Kelvin is 1/273.16 part of the thermodynamic temperature of the triple point of water.
The symbol T is used to denote temperature expressed in Kelvin, and t in degrees Celsius. The transition is made according to the formula: T=t+ 273.16. A degree Celsius is equal to one Kelvin (both units are eligible for use).
The unit of luminous intensity is candela (cd)
Luminous intensity is a quantity that characterizes the glow of a source in a certain direction, equal to the ratio of the luminous flux to the small solid angle in which it propagates.
Candela is equal to the luminous intensity in given direction a source emitting monochromatic radiation with a frequency of 540·10 12 Hz, the energy intensity of which in this direction is 1/683 (W/sr) (Watts per steradian).
The error in reproducing a unit with a standard is 1·10 -3 cd.
The unit of quantity of a substance is the mole.
A mole is equal to the amount of substance in a system containing the same number of structural elements as there are atoms in C12 carbon weighing 0.012 kg.
When using a mole, the structural elements must be specified and can be atoms, molecules, ions, electrons, or specified groups of particles.
Additional SI units
The international system includes two additional units - for measuring plane and solid angles. They cannot be basic, since they are dimensionless quantities. Assigning an independent dimension to an angle would lead to the need to change the mechanics equations related to rotational and curvilinear motion. However, they are not derivatives, since they do not depend on the choice of basic units. Therefore, these units are included in the SI as additional ones necessary for the formation of some derived units - angular velocity, angular acceleration, etc.
The unit of plane angle is radian (rad)
A radian is equal to the angle between two radii of a circle, the length of the arc between which is equal to the radius.
The state primary standard of the radian consists of a 36-sided prism and a standard goniometric autocollimation installation with a division value of the reading devices of 0.01’’. The reproduction of the plane angle unit is carried out by the calibration method, based on the fact that the sum of all central angles of a polyhedral prism is equal to 2π rad.
The unit of solid angle is steradian (sr)
The steradian is equal to the solid angle with its vertex at the center of the sphere, cutting out the area on the surface of the sphere, equal to the area square with a side equal to the radius of the sphere.
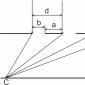
The solid angle is measured by determining the plane angles at the vertex of the cone. The solid angle 1ср corresponds to a flat angle 65 0 32’. For recalculation use the formula:
where Ω is the solid angle in sr; α is the plane angle at the vertex in degrees.
The solid angle π corresponds to a plane angle of 120 0, and the solid angle 2π corresponds to a plane angle of 180 0.
Usually angles are measured in degrees - this is more convenient.
Advantages of SI
It is universal, that is, it covers all measurement areas. With its implementation, you can abandon all other unit systems.
It is coherent, that is, a system in which the derived units of all quantities are obtained using equations with numerical coefficients equal to the dimensionless unit (the system is coherent and consistent).
The units in the system are unified (instead of a number of units of energy and work: kilogram-force-meter, erg, calorie, kilowatt-hour, electron-volt, etc. - one unit for measuring work and all types of energy - joule).
There is a clear distinction between units of mass and force (kg and N).
Disadvantages of SI
Not all units have a size convenient for practical use: the pressure unit Pa is a very small value; unit of electrical capacitance F is a very large value.
Inconvenience of measuring angles in radians (degrees are easier to perceive)
Many derived quantities do not yet have their own names.
Thus, the adoption of SI is the next and very important step in the development of metrology, a step forward in improving systems of units of physical quantities.
- 1 General information
- 2 History
- 3 SI units
- 3.1 Basic units
- 3.2 Derived units
- 4 Non-SI units
- Consoles
General information
The SI system was adopted by the XI General Conference on Weights and Measures, and some subsequent conferences made a number of changes to the SI.
The SI system defines seven main And derivatives units of measurement, as well as a set of . Standard abbreviations for units of measurement and rules for recording derived units have been established.
In Russia, GOST 8.417-2002 is in force, which prescribes the mandatory use of SI. It lists the units of measurement, gives their Russian and international names and establishes the rules for their use. According to these rules, only international designations are allowed to be used in international documents and on instrument scales. In internal documents and publications, you can use either international or Russian designations (but not both at the same time).
Basic units: kilogram, meter, second, ampere, kelvin, mole and candela. Within the SI framework, these units are considered to have independent dimensions, that is, none of the basic units can be obtained from the others.
Derived units are obtained from the basic ones using algebraic operations such as multiplication and division. Some of the derived units in the SI System are given their own names.
Consoles can be used before names of units of measurement; they mean that a unit of measurement must be multiplied or divided by a certain integer, a power of 10. For example, the prefix “kilo” means multiplying by 1000 (kilometer = 1000 meters). SI prefixes are also called decimal prefixes.
Story
The SI system is based on the metric system of measures, which was created by French scientists and was first widely introduced after the Great French Revolution. Before the introduction of the metric system, units of measurement were chosen randomly and independently of each other. Therefore, conversion from one unit of measurement to another was difficult. In addition, different units of measurement were used in different places, sometimes with the same names. The metric system was supposed to become a convenient and uniform system of measures and weights.
In 1799, two standards were approved - for the unit of length (meter) and for the unit of weight (kilogram).
In 1874, the GHS system was introduced, based on three units of measurement - centimeter, gram and second. Decimal prefixes from micro to mega were also introduced.
In 1889, the 1st General Conference on Weights and Measures adopted a system of measures similar to the GHS, but based on the meter, kilogram and second, since these units were considered more convenient for practical use.
Subsequently, basic units were introduced for measuring physical quantities in the field of electricity and optics.
In 1960, the XI General Conference on Weights and Measures adopted a standard that was first called the International System of Units (SI).
In 1971, the IV General Conference on Weights and Measures amended the SI, adding, in particular, a unit for measuring the amount of a substance (mole).
SI is now accepted as the legal system of units of measurement by most countries in the world and is almost always used in the scientific field (even in countries that have not adopted SI).
SI units
There is no dot after the designations of SI units and their derivatives, unlike usual abbreviations.
Basic units
| Magnitude | Unit | Designation | ||
|---|---|---|---|---|
| Russian name | international name | Russian | international | |
| Length | meter | meter (meter) | m | m |
| Weight | kilogram | kilogram | kg | kg |
| Time | second | second | With | s |
| Electric current strength | ampere | ampere | A | A |
| Thermodynamic temperature | kelvin | kelvin | TO | K |
| The power of light | candela | candela | cd | CD |
| Quantity of substance | mole | mole | mole | mol |
Derived units
Derived units can be expressed in terms of base units using the mathematical operations of multiplication and division. Some of the derived units are given their own names for convenience; such units can also be used in mathematical expressions to form other derived units.
The mathematical expression for a derived unit of measurement follows from the physical law by which this unit of measurement is defined or the definition of the physical quantity for which it is introduced. For example, speed is the distance a body travels per unit time. Accordingly, the unit of measurement for speed is m/s (meter per second).
Often the same unit of measurement can be written in different ways, using a different set of base and derived units (see, for example, the last column in the table ). However, in practice, established (or simply generally accepted) expressions are used, which the best way reflect physical meaning measured quantity. For example, to write the value of a moment of force, you should use N×m, and you should not use m×N or J.
| Magnitude | Unit | Designation | Expression | ||
|---|---|---|---|---|---|
| Russian name | international name | Russian | international | ||
| Flat angle | radian | radian | glad | rad | m×m -1 = 1 |
| Solid angle | steradian | steradian | Wed | sr | m 2 ×m -2 = 1 |
| Temperature in Celsius | degrees Celsius | °C | degree Celsius | °C | K |
| Frequency | hertz | hertz | Hz | Hz | s -1 |
| Force | newton | newton | N | N | kg×m/s 2 |
| Energy | joule | joule | J | J | N×m = kg×m 2 /s 2 |
| Power | watt | watt | W | W | J/s = kg × m 2 / s 3 |
| Pressure | pascal | pascal | Pa | Pa | N/m 2 = kg? m -1 ? s 2 |
| Light flow | lumen | lumen | lm | lm | kd×sr |
| Illumination | luxury | lux | OK | lx | lm/m 2 = cd×sr×m -2 |
| Electric charge | pendant | coulomb | Cl | C | А×с |
| Potential difference | volt | volt | IN | V | J/C = kg×m 2 ×s -3 ×A -1 |
| Resistance | ohm | ohm | Ohm | Ω | V/A = kg×m 2 ×s -3 ×A -2 |
| Capacity | farad | farad | F | F | C/V = kg -1 ×m -2 ×s 4 ×A 2 |
| Magnetic flux | weber | weber | Wb | Wb | kg×m 2 ×s -2 ×A -1 |
| Magnetic induction | tesla | tesla | Tl | T | Wb/m 2 = kg × s -2 × A -1 |
| Inductance | Henry | Henry | Gn | H | kg×m 2 ×s -2 ×A -2 |
| Electrical conductivity | Siemens | siemens | Cm | S | Ohm -1 = kg -1 ×m -2 ×s 3 A 2 |
| Radioactivity | becquerel | becquerel | Bk | Bq | s -1 |
| Absorbed dose of ionizing radiation | Gray | gray | Gr | Gy | J/kg = m 2 / s 2 |
| Effective dose of ionizing radiation | sievert | sievert | Sv | Sv | J/kg = m 2 / s 2 |
| Catalyst activity | rolled | catal | cat | kat | mol×s -1 |
Units not included in the SI System
Some units of measurement not included in the SI System are, by decision of the General Conference on Weights and Measures, “allowed for use in conjunction with SI.”
| Unit | International name | Designation | Value in SI units | |
|---|---|---|---|---|
| Russian | international | |||
| minute | minute | min | min | 60 s |
| hour | hour | h | h | 60 min = 3600 s |
| day | day | days | d | 24 h = 86,400 s |
| degree | degree | ° | ° | (P/180) glad |
| arcminute | minute | ′ | ′ | (1/60)° = (P/10,800) |
| arcsecond | second | ″ | ″ | (1/60)′ = (P/648,000) |
| liter | liter (liter) | l | l, L | 1 dm 3 |
| ton | tons | T | t | 1000 kg |
| neper | neper | Np | Np | |
| white | bel | B | B | |
| electron-volt | electronvolt | eV | eV | 10 -19 J |
| atomic mass unit | unified atomic mass unit | A. eat. | u | =1.49597870691 -27 kg |
| astronomical unit | astronomical unit | A. e. | ua | 10 11 m |
| nautical mile | nautical mile | mile | 1852 m (exactly) | |
| node | knot | bonds | 1 nautical mile per hour = (1852/3600) m/s | |
| ar | are | A | a | 10 2 m 2 |
| hectare | hectare | ha | ha | 10 4 m 2 |
| bar | bar | bar | bar | 10 5 Pa |
| angstrom | ångström | Å | Å | 10 -10 m |
| barn | barn | b | b | 10 -28 m 2 |
According to the definition approved by the XI General Conference on Weights and Measures, which adopted the SI system as the main mechanical unit The unit of mass adopted is the kilogram. The definition of kilogram is given as follows:
The unit of mass - the kilogram - is the mass of a substance equal to the mass of the prototype kilogram.
The prototype of the kilogram is a cylinder made of an alloy of 90% platinum and 10% iridium with a diameter of about 39 mm and the same height, located at the International Bureau of Weights and Measures in Sevres near Paris. The choice of this alloy ensures high storage qualities: chemical resistance, uniformity. The alloy is easy to polish and easy to clean. Due to its high density of 21.5 g/cm 3, it has the disadvantage that separation of small parts from it leads to a large change in mass. For this reason, copies of mass standards (secondary standards of various ranks) are usually made of steel or brass.
To ensure the uniformity of mass measurements during the establishment and approval of the kilogram prototype, many copies were made. The mass of prototypes was provided with distinction at the level of 10 -8 relative error. The prototypes were certified by the International Bureau of Weights and Measures. An error was assigned to each specimen. Possible fluctuations in the mass of the prototypes did not exceed 25 μg, which corresponds to a relative error of 2.5 × 10 -8. Prototype No. 12 was sent to Russia as a party to the Meter Convention in 1889, which is stored to this day at the All-Russian Research Institute named after. DI. Mendeleev (formerly the Main Chamber of Weights and Measures of Russia) in St. Petersburg.
Initially, the prototype mass had to coincide with the mass of one cubic decimeter of water at its highest density at a temperature of T = 3.98 ° C and a pressure of 101325 Pa. However, then the maximum density of water was found to be 0.999972 g/cm 3, i.e. the prototype mass turned out to be 28 micrograms more than it was intended. This would affect the definition of the unit of volume if it were introduced as the volume of one milliliter of water. With a known prototype mass of a kilogram, a unit of volume can be defined as the volume of 1000 g of water at its highest density and normal pressure. A unit defined in this way would correspond to the derived SI unit of volume as
The International System of Units (SI) is not set for everyone at all times. It has already been indicated that many countries use a different system of measures. Methods physical measurements are also constantly being improved. It is for this reason that a number of quantities have been redefined, for example, meter, candela. Ampere. For almost all basic units of the SI system, new definitions have been adopted based on physical phenomena, characterized by constancy and immunity to external influences. This makes it possible to create so-called “natural” or “imperishable” standards. Such standards were created for the basic units: length - meter, time - second, current - Ampere, thermodynamic temperature - Kelvin, luminous intensity - candela. The search for the same standard for a unit of mass - the kilogram - has not yet been successful. The accuracy achieved with the existing kilogram standard is very high and so far satisfies all practical needs. Nevertheless, with the entry of man into space, with the exploration of the World Ocean, etc., for many needs in measurement technology, it is desirable to have a natural standard of mass. The search for the possibility of replacing an artificial mass standard is now designated by metrologists as one of the most relevant scientific and practical problems.
One of the ways to solve this problem is the possibility of combining the problems of creating and storing standards for a unit of quantity of a substance and a unit of mass - the mole and the kilogram. To do this, it is necessary to create an accurate means of measuring the amount of matter with a range of changes in magnitude of 23 - 25 orders of magnitude, which corresponds to both the detection of individual particles and macroscopic measurements of the amount of matter that could be taken as a standard of inertial or gravitational mass.
How was the meter determined?In the 17th century, with the development of science in Europe, calls began to be increasingly heard to introduce a universal measure or Catholic meter. It would be a decimal measure based on a natural phenomenon, and independent of the decrees of the person in power. Such a measure would replace the many different systems of measures that existed at that time.
The British philosopher John Wilkins proposed taking the length of a pendulum as a unit of length, the half-period of which would be equal to one second. However, depending on the location of measurements, the value was different. French astronomer Jean Richet established this fact during his trip to South America (1671 - 1673).
In 1790, Minister Talleyrand proposed measuring the standard length by placing a pendulum at a strictly established latitude between Bordeaux and Grenoble - 45° north latitude. As a result, on May 8, 1790, the French National Assembly decided that the meter is the length of a pendulum with a half-period of oscillation at a latitude of 45° equal to 1 s. According to today's SI, that meter would be equal to 0.994 m. This definition, however, did not suit the scientific community.
On March 30, 1791, the French Academy of Sciences accepted a proposal to establish a standard meter as part of the Paris meridian. The new unit was to be one ten-millionth of the distance from the equator to the North Pole, that is, one ten-millionth of a quarter of the circumference of the Earth, measured along the Paris meridian. This became known as the “Genuine and Definitive Meter.”
April 7, 1795 National Convention adopted a law introducing the metric system in France and instructed commissioners, which included S. O. Coulon, J. L. Lagrange, P.-S. Laplace and other scientists experimentally determined units of length and mass.
In the period from 1792 to 1797, by decision of the revolutionary Convention, the French scientists Delambre (1749-1822) and Mechain (1744-1804) measured the arc of the Paris meridian with a length of 9 ° 40 "from Dunkirk to Barcelona in 6 years , laying a chain of 115 triangles across all of France and part of Spain.
Subsequently, however, it turned out that due to incorrect consideration of the polar compression of the Earth, the standard turned out to be 0.2 mm shorter. Thus, the meridian length of 40,000 km is only approximate. The first prototype of a brass meter standard, however, was made in 1795. It should be noted that the unit of mass (the kilogram, the definition of which was based on the mass of one cubic decimeter of water), was also tied to the definition of the meter.
History of the formation of the SI system
On June 22, 1799, two platinum standards were made in France - a standard meter and a standard kilogram. This date can rightly be considered the beginning of the development of the current SI system.
In 1832, Gauss created the so-called absolute system units, taking three basic units: the unit of time - the second, the unit of length - the millimeter, and the unit of mass - the gram, because using these very units the scientist was able to measure the absolute value magnetic field Earth (this system is called the Gaussian GHS).
In the 1860s, under the influence of Maxwell and Thomson, the requirement was formulated that basic and derived units must be consistent with each other. As a result, the GHS system was introduced in 1874, while prefixes were also allocated to designate submultiples and multiples of units from micro to mega.
In 1875, representatives of 17 states, including Russia, the USA, France, Germany, Italy, signed the Metric Convention, according to which the International Bureau of Measures, the International Committee of Measures were established and the regular convening of the General Conference on Weights and Measures (GCPM) began to operate. . At the same time, work began on the development of an international standard of the kilogram and a standard of the meter.
In 1889, at the first CGPM conference, the MKS system was adopted, based on the meter, kilogram and second, similar to the GHS, but the MKS units were seen as more acceptable due to the convenience of practical use. Units for optics and electricity will be introduced later.
In 1948, by order of the French government and the International Union of Theoretical and Applied Physics, the Ninth General Conference on Weights and Measures instructed the International Committee on Weights and Measures to propose, in order to unify the system of units of measurement, its ideas for creating a unified system of units of measurement, which could be accepted by all member states of the Meter Convention.
As a result, in 1954, at the tenth CGPM, the following six units were proposed and adopted: meter, kilogram, second, ampere, Kelvin and candela. In 1956, the system received the name “Système International d’Unités” - the international system of units. In 1960, a standard was adopted, which for the first time was called the “International System of Units”, and the abbreviation “SI” was assigned. The basic units remain the same six units: meter, kilogram, second, ampere, Kelvin and candela. (The Russian abbreviation “SI” can be deciphered as “International System”).
In 1963 in the USSR, according to GOST 9867-61 “International System of Units”, SI was adopted as the preferred one for regions National economy, in science and technology, as well as for teaching in educational institutions.
In 1968, at the thirteenth CGPM, the unit “degree Kelvin” was replaced by “kelvin”, and the designation “K” was also adopted. In addition, a new definition of a second was adopted: a second is a time interval equal to 9,192,631,770 periods of radiation corresponding to the transition between two hyperfine levels of the ground quantum state of the cesium-133 atom. In 1997, a clarification will be adopted, according to which this time interval refers to the cesium-133 atom at rest at 0 K.
In 1971, at the 14th CGPM, another basic unit “mole” was added - a unit of quantity of a substance. A mole is the amount of substance in a system containing the same number of structural elements as there are atoms in carbon-12 weighing 0.012 kg. When using a mole, the structural elements must be specified and can be atoms, molecules, ions, electrons and other particles or specified groups of particles.
In 1979, the 16th CGPM adopted a new definition for the candela. Candela is the luminous intensity in a given direction of a source emitting monochromatic radiation with a frequency of 540·1012 Hz, the luminous energy intensity of which in this direction is 1/683 W/sr (watt per steradian).
In 1983, a new definition of the meter was given at the 17th CGPM. A meter is the length of a path passable by light in a vacuum in (1/299,792,458) seconds.
In 2009, the Government of the Russian Federation approved the “Regulations on units of quantities allowed for use in Russian Federation”, and in 2015 it was amended to eliminate the “expiration date” of some non-system units.
Purpose of the SI system and its role in physics
Today, the international system of physical quantities SI is accepted throughout the world, and is used more than other systems both in science and technology, and in the everyday life of people - it is a modern version of the metric system.
Most countries use SI units in technology, even if Everyday life use traditional units for these territories. In the USA, for example, customary units are defined in terms of SI units using fixed coefficients.
| Magnitude | Designation | ||
| Russian name | Russian | international | |
| Flat angle | radian | glad | rad |
| Solid angle | steradian | Wed | sr |
| Celsius temperature | degrees Celsius | o C | o C |
| Frequency | hertz | Hz | Hz |
| Force | newton | N | N |
| Energy | joule | J | J |
| Power | watt | W | W |
| Pressure | pascal | Pa | Pa |
| Light flow | lumen | lm | lm |
| Illumination | luxury | OK | lx |
| Electric charge | pendant | Cl | C |
| Potential difference | volt | IN | V |
| Resistance | ohm | Ohm | Ω |
| Electrical capacity | farad | F | F |
| Magnetic flux | weber | Wb | Wb |
| Magnetic induction | tesla | Tl | T |
| Inductance | Henry | Gn | H |
| Electrical conductivity | Siemens | Cm | S |
| Radioactive source activity | becquerel | Bk | Bq |
| Absorbed dose of ionizing radiation | gray | Gr | Gy |
| Effective dose of ionizing radiation | sievert | Sv | Sv |
| Catalyst activity | rolled | cat | kat |
Exhaustive detailed description the SI system is officially presented in the SI Brochure published since 1970 and in its supplement; these documents are published on the official website of the International Bureau of Weights and Measures. Since 1985, these documents have been issued in English and French, and are always translated into a number of languages of the world, although official language document - French.
The precise official definition of the SI system is formulated as follows: “The International System of Units (SI) is a system of units based on the International System of Units, together with the names and symbols, as well as a set of prefixes and their names and symbols, together with the rules for their application, adopted by the General Conference according to weights and measures (CGPM)".
The SI system defines seven basic units of physical quantities and their derivatives, as well as their prefixes. Standard abbreviations for unit designations and rules for writing derivatives are regulated. There are, as before, seven basic units: kilogram, meter, second, ampere, kelvin, mole, candela. Basic units have independent dimensions and cannot be derived from other units.
As for derived units, they can be obtained on the basis of the basic ones by performing mathematical operations such as division or multiplication. Some of the derived units, such as “radian”, “lumen”, “coulomb”, have their own names.
Before the name of the unit, you can use a prefix, such as a millimeter - a thousandth of a meter, and a kilometer - a thousand meters. The prefix means that one must be divided or multiplied by a whole number that is a specific power of ten.
Since 1963, in the USSR (GOST 9867-61 “International System of Units”), in order to unify units of measurement in all fields of science and technology, the international (international) system of units (SI, SI) has been recommended for practical use - this is a system of units of measurement of physical quantities , adopted by the XI General Conference on Weights and Measures in 1960. It is based on 6 basic units (length, mass, time, electric current, thermodynamic temperature and luminous intensity), as well as 2 additional units (plane angle, solid angle) ; all other units given in the table are their derivatives. The adoption of a unified international system of units for all countries is intended to eliminate the difficulties associated with the translation of numerical values of physical quantities, as well as various constants from any one currently operating system (GHS, MKGSS, ISS A, etc.) into another.
| Name of quantity | Units; SI values | Designations | |
|---|---|---|---|
| Russian | international | ||
| I. Length, mass, volume, pressure, temperature | |||
| Meter is a measure of length, numerically equal to the length of the international standard meter; 1 m=100 cm (1·10 2 cm)=1000 mm (1·10 3 mm) |
m | m | |
| Centimeter = 0.01 m (1·10 -2 m) = 10 mm | cm | cm | |
| Millimeter = 0.001 m (1 10 -3 m) = 0.1 cm = 1000 μm (1 10 3 μm) | mm | mm | |
| Micron (micrometer) = 0.001 mm (1·10 -3 mm) = 0.0001 cm (1·10 -4 cm) = 10,000 |
mk | μ | |
| Angstrom = one ten-billionth of a meter (1·10 -10 m) or one hundred-millionth of a centimeter (1·10 -8 cm) | Å | Å | |
| Weight | The kilogram is the basic unit of mass in the metric system of measures and the SI system, numerically equal to the mass of the international standard kilogram; 1 kg=1000 g |
kg | kg |
| Gram=0.001 kg (1·10 -3 kg) |
G | g | |
| Ton= 1000 kg (1 10 3 kg) | T | t | |
| Centner = 100 kg (1 10 2 kg) |
ts | ||
| Carat - a non-systemic unit of mass, numerically equal to 0.2 g | ct | ||
| Gamma = one millionth of a gram (1 10 -6 g) | γ | ||
| Volume | Liter = 1.000028 dm 3 = 1.000028 10 -3 m 3 | l | l |
| Pressure | Physical, or normal, atmosphere - pressure balanced by a mercury column 760 mm high at a temperature of 0° = 1.033 atm = = 1.01 10 -5 n/m 2 = 1.01325 bar = 760 torr = 1.033 kgf/cm 2 |
atm | atm |
| Technical atmosphere - pressure equal to 1 kgf/cmg = 9.81 10 4 n/m 2 = 0.980655 bar = 0.980655 10 6 dynes/cm 2 = 0.968 atm = 735 torr | at | at | |
| Millimeter of mercury = 133.32 n/m 2 | mmHg Art. | mm Hg | |
| Tor is the name of a non-systemic unit of pressure measurement equal to 1 mm Hg. Art.; given in honor of the Italian scientist E. Torricelli | torus | ||
| Bar - unit of atmospheric pressure = 1 10 5 n/m 2 = 1 10 6 dynes/cm 2 | bar | bar | |
| Pressure (sound) | Bar is a unit of sound pressure (in acoustics): bar - 1 dyne/cm2; Currently, a unit with a value of 1 n/m 2 = 10 dynes/cm 2 is recommended as a unit of sound pressure |
bar | bar |
| Decibel is a logarithmic unit of measurement of excess sound pressure level, equal to 1/10 of the unit of measurement of excess sound pressure - bela | dB | db | |
| Temperature | Degree Celsius; temperature in °K (Kelvin scale), equal to temperature in °C (Celsius scale) + 273.15 °C | °C | °C |
| II. Force, power, energy, work, amount of heat, viscosity | |||
| Force | Dyna is a unit of force in the CGS system (cm-g-sec.), in which an acceleration of 1 cm/sec 2 is imparted to a body with a mass of 1 g; 1 din - 1·10 -5 n | ding | dyn |
| Kilogram-force is a force that imparts an acceleration to a body with a mass of 1 kg equal to 9.81 m/sec 2 ; 1kg=9.81 n=9.81 10 5 din | kg, kgf | ||
| Power | Horsepower =735.5 W | l. With. | HP |
| Energy | Electron-volt is the energy that an electron acquires when moving in an electric field in a vacuum between points with a potential difference of 1 V; 1 eV = 1.6·10 -19 J. It is allowed to use multiple units: kiloelectron-volt (Kv) = 10 3 eV and megaelectron-volt (MeV) = 10 6 eV. In modern times, particle energy is measured in Bev - billions (billions) eV; 1 Bzv=10 9 eV |
ev | eV |
| Erg=1·10 -7 j; The erg is also used as a unit of work, numerically equal to the work done by a force of 1 dyne along a path of 1 cm | erg | erg | |
| Job | Kilogram-force-meter (kilogrammometer) is a unit of work numerically equal to the work done by a constant force of 1 kg when moving the point of application of this force a distance of 1 m in its direction; 1 kGm = 9.81 J (at the same time kGm is a measure of energy) | kGm, kgf m | kGm |
| Quantity of heat | Calorie is an off-system unit of measurement of the amount of heat equal to the amount of heat required to heat 1 g of water from 19.5 ° C to 20.5 ° C. 1 cal = 4.187 J; common multiple unit kilocalorie (kcal, kcal), equal to 1000 cal | feces | cal |
| Viscosity (dynamic) | Poise is a unit of viscosity in the GHS system of units; viscosity at which in a layered flow with a velocity gradient equal to 1 sec -1 per 1 cm 2 of the layer surface, a viscous force of 1 dyne acts; 1 pz = 0.1 n sec/m 2 | pz | P |
| Viscosity (kinematic) | Stokes is a unit of kinematic viscosity in the CGS system; equal to the viscosity of a liquid having a density of 1 g/cm 3 that resists a force of 1 dyne to the mutual movement of two layers of liquid with an area of 1 cm 2 located at a distance of 1 cm from each other and moving relative to each other at a speed of 1 cm per second | st | St |
| III. Magnetic flux, magnetic induction, magnetic field strength, inductance, electrical capacitance | |||
| Magnetic flux | Maxwell is a unit of measurement of magnetic flux in the CGS system; 1 μs is equal to the magnetic flux passing through an area of 1 cm 2 located perpendicular to the magnetic field induction lines, with an induction equal to 1 gf; 1 μs = 10 -8 Wb (Weber) - units magnetic current in the SI system | mks | Mx |
| Magnetic induction | Gauss is a unit of measurement in the GHS system; 1 gf is the induction of such a field in which a straight conductor 1 cm long, located perpendicular to the field vector, experiences a force of 1 dyne if a current of 3 10 10 CGS units flows through this conductor; 1 gs=1·10 -4 tl (tesla) | gs | Gs |
| Magnetic field strength | Oersted is a unit of magnetic field strength in the CGS system; one oersted (1 oe) is taken to be the intensity at a point in the field at which a force of 1 dyne (dyn) acts on 1 electromagnetic unit of the amount of magnetism; 1 e=1/4π 10 3 a/m |
uh | Oe |
| Inductance | Centimeter is a unit of inductance in the CGS system; 1 cm = 1·10 -9 g (Henry) | cm | cm |
| Electrical capacity | Centimeter - unit of capacity in the CGS system = 1·10 -12 f (farads) | cm | cm |
| IV. Luminous intensity, luminous flux, brightness, illumination | |||
| The power of light | A candle is a unit of luminous intensity, the value of which is taken such that the brightness of the full emitter at the solidification temperature of platinum is equal to 60 sv per 1 cm2 | St. | CD |
| Light flow | Lumen is a unit of luminous flux; 1 lumen (lm) is emitted within a solid angle of 1 ster from a point source of light having a luminous intensity of 1 light in all directions | lm | lm |
| Lumen-second - corresponds to the light energy generated by a luminous flux of 1 lm emitted or perceived in 1 second | lm sec | lm·sec | |
| A lumen hour is equal to 3600 lumen seconds | lm h | lm h | |
| Brightness | Stilb is a unit of brightness in the CGS system; corresponds to the brightness of a flat surface, 1 cm 2 of which gives in a direction perpendicular to this surface a luminous intensity equal to 1 ce; 1 sb=1·10 4 nits (nit) (SI unit of brightness) | Sat | sb |
| Lambert is a non-systemic unit of brightness, derived from stilbe; 1 lambert = 1/π st = 3193 nt | |||
| Apostilbe = 1/π s/m 2 | |||
| Illumination | Phot - unit of illumination in the SGSL system (cm-g-sec-lm); 1 photo corresponds to the illumination of a surface of 1 cm2 with a uniformly distributed luminous flux of 1 lm; 1 f=1·10 4 lux (lux) | f | ph |
| V. Radiation intensity and dose | |||
| Intensity | Curie is the basic unit of measurement of the intensity of radioactive radiation, the curie corresponding to 3.7·10 10 decays per 1 second. any radioactive isotope |
curie | C or Cu |
| millicurie = 10 -3 curies, or 3.7 10 7 acts of radioactive decay in 1 second. | mcurie | mc or mCu | |
| microcurie= 10 -6 curie | mccurie | μC or μCu | |
| Dose | X-ray - the number (dose) of X-rays or γ-rays, which in 0.001293 g of air (i.e. in 1 cm 3 of dry air at t° 0° and 760 mm Hg) causes the formation of ions carrying one electrostatic unit of quantity of electricity of each sign; 1 p causes the formation of 2.08 10 9 pairs of ions in 1 cm 3 of air | R | r |
| milliroentgen = 10 -3 p | mr | mr | |
| microroentgen = 10 -6 p | microdistrict | μr | |
| Rad - the unit of absorbed dose of any ionizing radiation is equal to rad 100 erg per 1 g of irradiated medium; when air is ionized by X-rays or γ-rays, 1 r is equal to 0.88 rad, and when tissue is ionized, almost 1 r is equal to 1 rad | glad | rad | |
| Rem (biological equivalent of an x-ray) - amount (dose) of any kind ionizing radiation, causing the same biological effect as 1 r (or 1 rad) of hard x-rays. Uneven biological effect with equal ionization different types radiation led to the need to introduce another concept: the relative biological effectiveness of radiation - RBE; the relationship between doses (D) and the dimensionless coefficient (RBE) is expressed as D rem = D rad RBE, where RBE = 1 for x-rays, γ-rays and β-rays and RBE = 10 for protons up to 10 MeV, fast neutrons and α - natural particles (according to the recommendation of the International Congress of Radiologists in Copenhagen, 1953) | reb, reb | rem | |
Note. Multiple and submultiple units of measurement, with the exception of units of time and angle, are formed by multiplying them by the appropriate power of 10, and their names are added to the names of the units of measurement. It is not allowed to use two prefixes to the name of the unit. For example, you cannot write millimicrowatt (mmkW) or micromicrofarad (mmf), but you must write nanowatt (nw) or picofarad (pf). Prefixes should not be applied to the names of such units that indicate a multiple or submultiple unit of measurement (for example, micron). To express the duration of processes and designate calendar dates of events, the use of multiple units of time is allowed.
The most important units of the International System of Units (SI)
Basic units
(length, mass, temperature, time, electric current, light intensity)
| Name of quantity | Designations | ||
|---|---|---|---|
| Russian | international | ||
| Length | Meter - length equal to 1650763.73 wavelengths of radiation in vacuum, corresponding to the transition between levels 2p 10 and 5d 5 of krypton 86 * |
m | m |
| Weight | Kilogram - mass corresponding to the mass of the international standard kilogram | kg | kg |
| Time | Second - 1/31556925.9747 part of a tropical year (1900)** | sec | S, s |
| Electric current strength | Ampere is the strength of a constant current, which, passing through two parallel straight conductors of infinite length and negligible circular cross-section, located at a distance of 1 m from each other in a vacuum, would cause between these conductors a force equal to 2 10 -7 N per meter length | A | A |
| The power of light | A candle is a unit of luminous intensity, the value of which is taken such that the brightness of a complete (absolutely black) emitter at the solidification temperature of platinum is equal to 60 sec per 1 cm 2 *** | St. | CD |
| Temperature (thermodynamic) | Degree Kelvin (Kelvin scale) is a unit of measurement of temperature on the thermodynamic temperature scale, in which the temperature of the triple point of water**** is set to 273.16° K | °K | °K |
** That is, a second is equal to the specified part of the time interval between two successive passages by the Earth in its orbit around the Sun of the point corresponding to the vernal equinox. This gives greater accuracy in determining the second than defining it as a part of the day, since the length of the day varies.
*** That is, the luminous intensity of a certain reference source emitting light at the melting temperature of platinum is taken as a unit. The old international candle standard is 1.005 of the new candle standard. Thus, within the limits of normal practical accuracy, their values can be considered identical.
**** Triple point - the temperature at which ice melts in the presence of saturated water vapor above it.
Additional and derived units
| Name of quantity | Units; their definition | Designations | |
|---|---|---|---|
| Russian | international | ||
| I. Plane angle, solid angle, force, work, energy, amount of heat, power | |||
| Flat angle | Radian - the angle between two radii of a circle, cutting out an arc on the circle, the length of which is equal to the radius | glad | rad |
| Solid angle | Steradian is a solid angle whose vertex is located at the center of the sphere and which cuts out an area on the surface of the sphere equal to the area of a square with a side equal to the radius of the sphere | erased | sr |
| Force | Newton is a force under the influence of which a body with a mass of 1 kg acquires an acceleration equal to 1 m/sec 2 | n | N |
| Work, energy, amount of heat | Joule is the work done by a constant force of 1 N acting on a body along a path of 1 m traveled by the body in the direction of the force. | j | J |
| Power | Watt - power at which in 1 second. 1 J of work done | W | W |
| II. Amount of electricity, electrical voltage, electrical resistance, electrical capacitance | |||
| The amount of electricity electric charge | Coulomb - the amount of electricity flowing through the cross-section of a conductor for 1 second. with strength direct current in 1 a | To | C |
| Electrical voltage, difference electrical potentials, electromotive force (EMF) | Volt is the voltage in a section of an electrical circuit through which 1 k of electricity passes through which 1 j of work is done. | V | V |
| Electrical resistance | Ohm - the resistance of a conductor through which, at a constant voltage at the ends of 1 V, a constant current of 1 A passes | ohm | Ω |
| Electrical capacity | Farad is the capacitance of a capacitor, the voltage between the plates of which changes by 1 V when charging it with an amount of electricity of 1 k. | f | F |
| III. Magnetic induction, magnetic flux, inductance, frequency | |||
| Magnetic induction | Tesla is the induction of a uniform magnetic field, which acts on a section of a straight conductor 1 m long, placed perpendicular to the direction of the field, with a force of 1 N when a direct current of 1 A passes through the conductor | tl | T |
| Magnetic induction flux | Weber - magnetic flux created by a uniform field with a magnetic induction of 1 T through an area of 1 m 2 perpendicular to the direction of the magnetic induction vector | wb | Wb |
| Inductance | Henry is the inductance of a conductor (coil) in which an emf of 1 V is induced when the current in it changes by 1 A in 1 second. | gn | H |
| Frequency | Hertz is the frequency of a periodic process in which in 1 sec. one oscillation occurs (cycle, period) | Hz | Hz |
| IV. Luminous flux, luminous energy, brightness, illumination | |||
| Light flow | Lumen is a luminous flux that gives within a solid angle of 1 ster a point source of light of 1 sv, emitting equally in all directions | lm | lm |
| Light energy | Lumen-second | lm sec | lm·s |
| Brightness | Nit - the brightness of the luminous plane, each square meter which gives in the direction perpendicular to the plane a luminous intensity of 1 light | nt | nt |
| Illumination | Lux - illumination created by a luminous flux of 1 lm with its uniform distribution over an area of 1 m2 | OK | lx |
| Lighting quantity | Lux second | lx sec | lx·s |
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0