A protein is a sequence of amino acids linked to each other by peptide bonds.
It is easy to imagine that the number of amino acids can be different: from at least two to any reasonable values. Biochemists agreed to consider that if the number of amino acids does not exceed 10, then such a compound is called a peptide; if from 10 or more amino acids - polypeptide. Polypeptides that can spontaneously form and maintain a certain spatial structure, which is called conformation, are referred to as proteins. Stabilization of such a structure is possible only when polypeptides reach a certain length (more than 40 amino acids); therefore, polypeptides with a molecular weight of more than 5,000 Da are usually considered proteins. (1Da is equal to 1/12 of a carbon isotope). Only having a certain spatial structure (native structure) can a protein perform its functions.
Protein size can be measured in daltons ( molecular mass), more often because of the relatively large size of the molecule in derived units - kilodaltons (kDa). Yeast proteins, on average, consist of 466 amino acids and have a molecular weight of 53 kDa. The largest protein currently known, titin, is a component of muscle sarcomeres; the molecular weight of its various isoforms varies from 3000 to 3700 kDa, it consists of 38,138 amino acids (in the human solius muscle).
protein structure
The three-dimensional structure of a protein is formed in the process of folding (from the English. folding-"folding"). A three-dimensional structure is formed as a result of the interaction of structures of lower levels.
There are four levels of protein structure:
Primary Structure- the sequence of amino acids in the polypeptide chain.
secondary structure- this is the placement in space of individual sections of the polypeptide chain.
The following are the most common types of protein secondary structure:
α-helices- tight turns around the long axis of the molecule, one turn is 3.6 amino acid residues, and the helix pitch is 0.54 nm (0.15 nm per amino acid residue), the helix is stabilized by hydrogen bonds between H and O peptide groups spaced apart 4 amino acid residues apart. The helix is built exclusively from one type of stereoisomers of amino acids (L). Although it can be either left-handed or right-handed, right-handed predominates in proteins. The spiral is broken by electrostatic interactions of glutamic acid, lysine, arginine. Closely spaced asparagine, serine, threonine, and leucine residues can sterically interfere with helix formation, proline residues cause chain bending and also disrupt the structure of the α-helix.
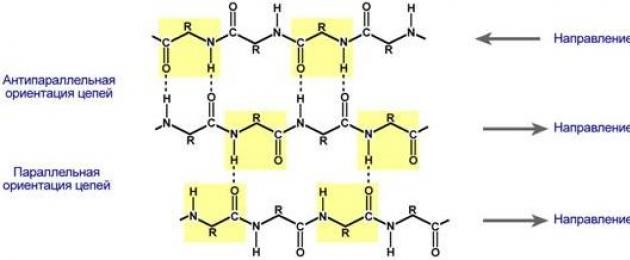
β-pleated layers- several zigzag polypeptide chains in which hydrogen bonds are formed between amino acids or different protein chains relatively distant from each other (0.347 nm per amino acid residue) in the primary structure, and not closely spaced, as is the case in the α-helix. These chains are usually directed with their N-terminals in opposite directions (anti-parallel orientation). For the formation of β-folded layers, the small sizes of the side groups of amino acids are important; glycine and alanine usually predominate.

Protein folding in the form of a β-folded layer
Disordered structures are the disordered arrangement of a protein chain in space.
The spatial structure of each protein is individual and is determined by its primary structure. However, a comparison of the conformations of proteins with different structures and functions revealed the presence of similar combinations of secondary structure elements in them. Such a specific order of formation of secondary structures is called the supersecondary structure of proteins. The supersecondary structure is formed due to interradical interactions.
Certain characteristic combinations of α-helices and β-structures are often referred to as "structural motifs". They have specific names: "α-helix-turn-α-helix", "α/β-barrel structure", "leucine zipper", "zinc finger", etc.
Tertiary structure - this is a way to place the entire polypeptide chain in space. Along with α-helices, β-folded layers, and supersecondary structures, the tertiary structure exhibits a disordered conformation, which can occupy a significant part of the molecule.

Schematic representation of protein folding into a tertiary structure.
Quaternary structure occurs in proteins that consist of several polypeptide chains (subunits, protomers or monomers), when the tertiary structures of these subunits are combined. For example, the hemoglobin molecule consists of 4 subunits. Supramolecular formations have a quaternary structure - multienzyme complexes that consist of several molecules of enzymes and coenzymes (pyruvate dehydrogenase), and isoenzymes (lactate dehydrogenase - LDH, creatine phosphokinase - CPK).
so. The spatial structure does not depend on the length of the polypeptide chain, but on the sequence of amino acid residues specific to each protein, as well as on the side radicals characteristic of the corresponding amino acids. The spatial three-dimensional structure or conformation of protein macromolecules is primarily formed by hydrogen bonds, hydrophobic interactions between non-polar side radicals of amino acids, and ionic interactions between oppositely charged side groups of amino acid residues. Hydrogen bonds play a huge role in the formation and maintenance of the spatial structure of the protein macromolecule.
As for hydrophobic interactions, they arise as a result of contact between non-polar radicals that are unable to break hydrogen bonds between water molecules, which is displaced to the surface of the protein globule. As the protein is synthesized, non-polar chemical groups are collected inside the globule, and polar ones are forced out onto its surface. Thus, a protein molecule can be neutral, positively charged, or negatively charged, depending on the pH of the solvent and the ionic groups in the protein. In addition, protein conformation is maintained by S-S covalent bonds formed between two cysteine residues. As a result of the formation of a native protein structure, many atoms located at remote sites of the polypeptide chain approach each other and, acting on each other, acquire new properties that are absent in individual amino acids or small polypeptides.
It is important to understand that folding - the folding of proteins (and other biomacromolecules) from an unfolded conformation into a "native" form - is a physicochemical process, as a result of which proteins in their natural "habitat" (solution, cytoplasm or membrane) acquire characteristics characteristic only of them. spatial arrangement and function.
Cells contain a number of catalytically inactive proteins, which, nevertheless, make a great contribution to the formation of spatial structures of proteins. These are the so-called chaperones. Chaperones aid in the correct assembly of the three-dimensional protein conformation by forming reversible, non-covalent complexes with the partially folded polypeptide chain, while inhibiting malformed bonds leading to the formation of functionally inactive protein structures. The list of functions inherent in chaperones includes the protection of molten (partially folded) globules from aggregation, as well as the transfer of newly synthesized proteins to various cell loci.
Chaperones are predominantly heat shock proteins, the synthesis of which increases sharply under stressful temperature exposure, so they are also called hsp (heat shock proteins). Families of these proteins are found in microbial, plant, and animal cells. The classification of chaperones is based on their molecular weight, which varies from 10 to 90 kDa. They are helper proteins in the processes of formation of the three-dimensional structure of proteins. Chaperones keep the newly synthesized polypeptide chain in an unfolded state, preventing it from folding into a form different from the native one, and provide conditions for the only correct, native protein structure.
In the process of protein folding, some conformations of the molecule are rejected at the stage of the molten globule. The degradation of such molecules is initiated by the protein ubiquitin.
Protein degradation via the ubiquitin pathway involves two main steps:
1) covalent attachment of ubiquitin to the protein to be degraded through the residue lysine, the presence of such a label in the protein is the primary sorting signal that directs the resulting conjugates to the proteasomes; in most cases, several ubiquitin molecules are attached to the protein, which are organized in the form of beads on a string .;
2) protein hydrolysis by the proteasome (the main function of the proteasome is the proteolytic degradation of unnecessary and damaged proteins to short peptides). Ubiquitin is deservedly called the “mark of death” for protein.
Domain? n protein? - element of the tertiary structure of the protein, which is a fairly stable and independent substructure of the protein, whose folding takes place independently of the other parts. The domain usually includes several elements of the secondary structure. Domains similar in structure are found not only in related proteins (for example, in the hemoglobins of different animals), but also in completely different proteins. A protein can have several domains, these regions can perform different functions in the same protein. Some enzymes and all immunoglobulins have a domain structure. Proteins with long polypeptide chains (more than 200 amino acid residues) often create domain structures.
Biological chemistry Lelevich Vladimir Valeryanovich
Levels of structural organization of proteins
Primary Structure- a strictly defined linear sequence of amino acids in the polypeptide chain.
The strategic principles for studying the primary structure of a protein have undergone significant changes with the development and improvement of the methods used. Three main stages in their development should be noted. The first stage begins with the classic work of F. Sanger (1953) on the establishment of the amino acid sequence of insulin, the second - with the widespread introduction of an automatic sequencer into the structural analysis of protein (the beginning of the 70s of the 20th century), the third - with the development of high-speed methods for analyzing the DNA nucleotide sequence ( early 1980s).
The primary structure of a protein is determined by:
1. The nature of the amino acids included in the molecule.
2. The relative amount of each amino acid.
3. A strictly defined sequence of amino acids in the polypeptide chain.
Preliminary studies before determining the primary structure of a protein
1. Protein Purification
2. Determination of molecular weight.
3. Determining the type and number of prosthetic groups (if the protein is conjugated).
4. Determination of the presence of intra- or intermolecular disulfide bonds. Usually, the presence of sulfhydryl groups in the native protein is determined simultaneously.
5. Pretreatment of proteins with the 4th structure for the purpose of dissociation of subunits, their isolation and subsequent study.
Stages of determining the primary structure of proteins and polypeptides
1. Determination of amino acid composition (hydrolysis, amino acid analyzer).
2. Identification of N- and C-terminal amino acids.
3. Cleavage of the polypeptide chain into fragments (trypsin, chymotrypsin, cyanogen bromide, hydroxylamine, etc.).
4. Determination of the amino acid sequence of peptide fragments (sequencer).
5. Cleavage of the original polypeptide chain by other means and determination of their amino acid sequence.
6. Establishing the order of arrangement of peptide fragments in overlapping areas (obtaining peptide maps).
Methods for determining N-terminal amino acids
1. Sanger's method.
2. Edman's method (implemented in the sequencer).
3. Reaction with dansyl chloride.
4. Method using aminopeptidase.
Methods for determination of C-terminal amino acids
1. Akabori method.
2. Method using carboxypeptidase.
3. Method using sodium borohydride.
General patterns regarding the amino acid sequence of proteins
1. There is no single unique sequence or group of partial sequences common to all proteins.
2. Proteins that perform different functions have different sequences.
3. Proteins with similar functions have similar sequences, but there is usually only a small degree of sequence matching.
4. The same proteins that perform the same functions, but isolated from different organisms, usually have a significant similarity in sequence.
5. The same proteins that perform the same functions and isolated from organisms of the same species almost always have exactly the same sequence.
The highest levels of protein structure, their biological activity are closely related and are actually determined by the amino acid sequence. I.e, primary structure is genetically determined and determines the individual properties of proteins, their species specificity; all subsequent structures are formed on its basis.
The secondary structure of a protein is the configuration of a polypeptide chain resulting from interactions between its functional groups.
Varieties of the secondary structure:
1. ?-spiral.
2. Folded sheet (?-structure).
3. Statistical tangle.
The first two varieties are an ordered arrangement, the third is an unordered one.
Super secondary structure of proteins.
Comparison of the conformations of proteins with different structure and functions revealed the presence of similar combinations of secondary structure elements in them. Such a specific order of formation of secondary structures is called a supersecondary structure. The supersecondary structure is formed due to interradical interactions.
Varieties of the super secondary structure of proteins:
1. Super secondary structure of the ?-barrel type. It really resembles a barrel, where each α-structure is located inside and is connected by a α-helical section of the chain located on the surface. It is characteristic of some enzymes - triose phosphate isomerase, pyruvate kinase.
2. Structural motif "?-helix - turn - ?-helix". Found in many DNA-binding proteins.
3. Super secondary structure in the form of a "zinc finger". It is also characteristic of DNA-binding proteins. "Zinc finger" - a protein fragment containing about 20 amino acids, in which the zinc atom is associated with four amino acid radicals: usually two cysteine residues and two histidine residues.
4. Super secondary structure in the form of a "leucine zipper". The combination of protomers or individual proteins into complexes is sometimes carried out using structural motifs called "leucine zipper". Histones are an example of such a combination of proteins. These are nuclear proteins, which include a large number of positively charged amino acids - arginine and lysine. Histone molecules are combined into complexes with the help of "leucine fasteners", despite the fact that all monomers have a strong positive charge.
By the presence of?-helices and?-structures, globular proteins can be divided into 4 categories:
The tertiary structure of a protein is the spatial orientation of the polypeptide chain or the way it is laid in a certain volume.
Depending on the shape of the tertiary structure, globular and fibrillar proteins are distinguished. In globular proteins, the α-helix often predominates, fibrillar proteins are formed on the basis of the β-structure.
In the stabilization of the tertiary structure of the globular protein, the following can take part:
1. hydrogen bonds of a spiral structure;
2. hydrogen bonds?-structures;
3. hydrogen bonds between radicals of side chains;
4. hydrophobic interactions between non-polar groups;
5. electrostatic interactions between oppositely charged groups;
6. disulfide bonds;
7. coordination bonds of metal ions.
The quaternary structure of a protein is a way of laying in space individual polypeptide chains that have the same (or different) primary, secondary or tertiary structure, and the formation of a single macromolecular formation in structural and functional respects.
The quaternary structure is characteristic of proteins consisting of several subunits. The interaction between complementary sites of subunits in the quaternary structure is carried out using hydrogen and ionic bonds, van der Waals forces, and hydrophobic interactions. Rarely, covalent bonds occur.
Advantages of subunit protein construction compared to a single long polypeptide chain.
First, the presence of a subunit structure makes it possible to “save” genetic material. For oligomeric proteins consisting of identical subunits, the size of the structural gene and, accordingly, the length of messenger RNA sharply decrease.
Secondly, with a relatively small chain size, the influence of random errors that can occur during the biosynthesis of protein molecules decreases. In addition, additional culling of "incorrect", erroneous polypeptides in the process of association of subunits into a single complex is possible.
Thirdly, the presence of a subunit structure in many proteins allows the cell to easily regulate their activity by shifting the association-dissociation equilibrium in one direction or another.
Finally, the subunit structure facilitates and accelerates the process of molecular evolution. Mutations that lead to only small conformational changes at the level of the tertiary structure due to the multiple amplification of these changes during the transition to the quaternary structure can contribute to the appearance of new properties in the protein.
From the book Biology [ Complete reference to prepare for the exam] author Lerner Georgy Isaakovich From the book The Missing Link author Edie MaitlandGenealogical tree(evidence for proteins) Family tree (evidence for proteins) Differences in the proteins of two species reflect evolutionary changes in these species after their separation from a common ancestor. The analysis shows that between chimpanzee blood serum albumins
From the book Conversations about Life author Galaktionov Stanislav GennadievichChapter 2. Molecular architecture of proteins Let's not hide it: having finished with the first chapter, the authors (and possibly the reader) experienced some relief. After all, its purpose was only to give the reader the information necessary to understand the following chapters,
From the book Evolution [Classic ideas in the light of new discoveries] authorThe Universe of Ancient Proteins Continues to Expand In 2010, Nature published an interesting paper on the evolutionary movement of proteins across fitness landscapes (Povolotskaya and Kondrashov, 2010). The authors of the work decided to compare the amino acid sequences of 572 ancient proteins,
From the book Genes and Development of the Body author Neifakh Alexander Alexandrovich4. Variants of the structural hypothesis So, several experimental data indicate the possibility of such structural changes that are preserved during mitosis and replication, can be transmitted in a series of cell generations and provide epigenetic
From the book Human Evolution. Book 1. Monkeys, bones and genes author Markov Alexander VladimirovichProtein Changes Those parts of the genome that code for proteins have changed surprisingly little. Differences in the amino acid sequences of proteins in humans and chimpanzees are significantly less than 1%, and of these few differences most of either does not have
From the book Biology. General biology. Grade 10. A basic level of author Sivoglazov Vladislav Ivanovich3. Levels of organization of living matter. Methods of biology Remember! What levels of organization of living matter do you know? What methods of scientific research do you know? Levels of organization of living matter. The world of living beings around us is a collection biological systems
From the book Anthropology and Concepts of Biology author Kurchanov Nikolai AnatolievichStructural and functional levels of life organization In biology, there are several structural and functional levels of organization of living matter. Molecular level. It is characterized by biochemical substances that make up a living organism. Cellular level.
From the book Biological Chemistry author Lelevich Vladimir ValeryanovichChapter 2. The structure and functions of proteins Proteins are high-molecular nitrogen-containing organic compounds, consisting of amino acids connected into polypeptide chains using peptide bonds, and having a complex structural organization. History of the study of proteins In 1728
From the author's bookFunctioning of Proteins Each individual protein, having a unique primary structure and conformation, also has a unique function that distinguishes it from all other proteins. A set of individual proteins performs many diverse and complex functions in the cell.
From the author's bookPost-translational changes in proteins Many proteins are synthesized in an inactive form (precursors) and, after merging with ribosomes, undergo post-synthetic structural modifications. These conformational and structural changes in polypeptide chains
From the author's bookLevels of study of metabolism Levels of study of metabolism: 1. The whole organism.2. Isolated organs (perfused).3. Sections of tissues.4. Cell cultures.5. Tissue homogenates.6. Isolated cell organelles.7. Molecular level (purified enzymes, receptors and
From the author's bookDigestion of proteins in the gastrointestinal tract Digestion of proteins begins in the stomach under the action of enzymes in the gastric juice. Up to 2.5 liters are released per day and it differs from other digestive juices in a strongly acidic reaction, due to the presence
From the author's bookCleavage of proteins in tissues It is carried out with the help of proteolytic lysosomal enzymes cathepsins. According to the structure of the active center, cysteine, serine, carboxyl and metalloprotein cathepsins are distinguished. The role of cathepsins: 1. creation of biologically active
From the author's bookThe role of the liver in the metabolism of amino acids and proteins The liver plays a central role in the metabolism of proteins and other nitrogen-containing compounds. It performs the following functions: 1. synthesis of specific plasma proteins: - in the liver is synthesized: 100% albumin, 75 - 90% ?-globulins, 50%
From the author's bookCharacteristics of blood serum proteins Proteins of the complement system - this system includes 20 proteins circulating in the blood in the form of inactive precursors. Their activation occurs under the action of specific substances with proteolytic activity.
Squirrels- high-molecular organic compounds, consisting of residues of α-amino acids.
IN protein composition includes carbon, hydrogen, nitrogen, oxygen, sulfur. Some proteins form complexes with other molecules containing phosphorus, iron, zinc and copper.
Proteins have a large molecular weight: egg albumin - 36,000, hemoglobin - 152,000, myosin - 500,000. For comparison: the molecular weight of alcohol is 46, acetic acid - 60, benzene - 78.
Amino acid composition of proteins
Squirrels- non-periodic polymers, the monomers of which are α-amino acids. Usually, 20 types of α-amino acids are called protein monomers, although more than 170 of them have been found in cells and tissues.
Depending on whether amino acids can be synthesized in the body of humans and other animals, there are: non-essential amino acids- can be synthesized essential amino acids- cannot be synthesized. Essential amino acids must be ingested with food. Plants synthesize all kinds of amino acids.
Depending on the amino acid composition, proteins are: complete- contain the entire set of amino acids; defective- some amino acids are absent in their composition. If proteins are made up of only amino acids, they are called simple. If proteins contain, in addition to amino acids, also a non-amino acid component (a prosthetic group), they are called complex. The prosthetic group can be represented by metals (metalloproteins), carbohydrates (glycoproteins), lipids (lipoproteins), nucleic acids (nucleoproteins).
Everything amino acids contain: 1) a carboxyl group (-COOH), 2) an amino group (-NH 2), 3) a radical or R-group (the rest of the molecule). The structure of the radical in different types of amino acids is different. Depending on the number of amino groups and carboxyl groups that make up amino acids, there are: neutral amino acids having one carboxyl group and one amino group; basic amino acids having more than one amino group; acidic amino acids having more than one carboxyl group.
Amino acids are amphoteric compounds, since in solution they can act as both acids and bases. In aqueous solutions, amino acids exist in different ionic forms.
Peptide bond
Peptides- organic substances consisting of amino acid residues connected by a peptide bond.
The formation of peptides occurs as a result of the condensation reaction of amino acids. When the amino group of one amino acid interacts with the carboxyl group of another, a covalent nitrogen-carbon bond arises between them, which is called peptide. Depending on the number of amino acid residues that make up the peptide, there are dipeptides, tripeptides, tetrapeptides etc. The formation of a peptide bond can be repeated many times. This leads to the formation polypeptides. At one end of the peptide there is a free amino group (it is called the N-terminus), and at the other end there is a free carboxyl group (it is called the C-terminus).

Spatial organization of protein molecules
The performance of certain specific functions by proteins depends on the spatial configuration of their molecules, in addition, it is energetically unfavorable for the cell to keep proteins in an expanded form, in the form of a chain, therefore, polypeptide chains undergo folding, acquiring a certain three-dimensional structure, or conformation. Allocate 4 levels spatial organization of proteins.
Primary structure of a protein- the sequence of amino acid residues in the polypeptide chain that makes up the protein molecule. The bond between amino acids is peptide.

If a protein molecule consists of only 10 amino acid residues, then the number of theoretically possible variants of protein molecules that differ in the order of alternation of amino acids is 10 20 . With 20 amino acids, you can make even more diverse combinations of them. About ten thousand different proteins have been found in the human body, which differ both from each other and from the proteins of other organisms.
It is the primary structure of the protein molecule that determines the properties of the protein molecules and its spatial configuration. The replacement of just one amino acid for another in the polypeptide chain leads to a change in the properties and functions of the protein. For example, the replacement of the sixth glutamine amino acid in the β-subunit of hemoglobin with valine leads to the fact that the hemoglobin molecule as a whole cannot perform its main function - oxygen transport; in such cases, a person develops a disease - sickle cell anemia.
secondary structure- ordered folding of the polypeptide chain into a spiral (looks like a stretched spring). The coils of the helix are strengthened by hydrogen bonds between carboxyl groups and amino groups. Almost all CO and NH groups take part in the formation of hydrogen bonds. They are weaker than peptide ones, but, repeating many times, they impart stability and rigidity to this configuration. At the level of the secondary structure, there are proteins: fibroin (silk, web), keratin (hair, nails), collagen (tendons).



Tertiary structure- packing of polypeptide chains into globules, resulting from the occurrence chemical bonds(hydrogen, ionic, disulfide) and the establishment of hydrophobic interactions between radicals of amino acid residues. The main role in the formation of the tertiary structure is played by hydrophilic-hydrophobic interactions. In aqueous solutions, hydrophobic radicals tend to hide from water, grouping inside the globule, while hydrophilic radicals tend to appear on the surface of the molecule as a result of hydration (interaction with water dipoles). In some proteins, the tertiary structure is stabilized by disulfide covalent bonds that form between the sulfur atoms of the two cysteine residues. At the level of the tertiary structure, there are enzymes, antibodies, some hormones.

Quaternary structure characteristic of complex proteins, the molecules of which are formed by two or more globules. Subunits are held in the molecule by ionic, hydrophobic, and electrostatic interactions. Sometimes, during the formation of a quaternary structure, disulfide bonds occur between subunits. The most studied protein with a quaternary structure is hemoglobin. It is formed by two α-subunits (141 amino acid residues) and two β-subunits (146 amino acid residues). Each subunit is associated with a heme molecule containing iron.
If for some reason the spatial conformation of proteins deviates from normal, the protein cannot perform its functions. For example, the cause of "mad cow disease" (spongiform encephalopathy) is an abnormal conformation of prions, the surface proteins of nerve cells.
Protein properties
The amino acid composition, the structure of the protein molecule determine its properties. Proteins combine basic and acidic properties determined by amino acid radicals: the more acidic amino acids in a protein, the more pronounced its acidic properties. The ability to give and attach H + determine buffer properties of proteins; one of the most powerful buffers is hemoglobin in erythrocytes, which maintains the pH of the blood at a constant level. There are soluble proteins (fibrinogen), there are insoluble proteins that perform mechanical functions (fibroin, keratin, collagen). There are chemically active proteins (enzymes), there are chemically inactive, resistant to various environmental conditions and extremely unstable.
External factors (heat, ultraviolet radiation, heavy metals and their salts, pH changes, radiation, dehydration)
can cause a violation of the structural organization of the protein molecule. The process of losing the three-dimensional conformation inherent in a given protein molecule is called denaturation. The cause of denaturation is the breaking of bonds that stabilize a particular protein structure. Initially, the weakest ties are torn, and when conditions become tougher, even stronger ones. Therefore, first the quaternary, then the tertiary and secondary structures are lost. A change in the spatial configuration leads to a change in the properties of the protein and, as a result, makes it impossible for the protein to perform its biological functions. If denaturation is not accompanied by the destruction of the primary structure, then it can be reversible, in this case, self-healing of the conformation characteristic of the protein occurs. Such denaturation is subjected, for example, to membrane receptor proteins. The process of restoring the structure of a protein after denaturation is called renaturation. If the restoration of the spatial configuration of the protein is impossible, then denaturation is called irreversible.
Functions of proteins
| Function | Examples and explanations |
|---|---|
| Construction | Proteins are involved in the formation of cellular and extracellular structures: they are part of cell membranes (lipoproteins, glycoproteins), hair (keratin), tendons (collagen), etc. |
| Transport | The blood protein hemoglobin attaches oxygen and transports it from the lungs to all tissues and organs, and from them to the lungs transfers carbon dioxide; The composition of cell membranes includes special proteins that provide an active and strictly selective transfer of certain substances and ions from the cell to the external environment and vice versa. |
| Regulatory | Protein hormones are involved in the regulation of metabolic processes. For example, the hormone insulin regulates blood glucose levels, promotes glycogen synthesis, and increases the formation of fats from carbohydrates. |
| Protective | In response to the penetration of foreign proteins or microorganisms (antigens) into the body, special proteins are formed - antibodies that can bind and neutralize them. Fibrin, formed from fibrinogen, helps to stop bleeding. |
| Motor | The contractile proteins actin and myosin provide muscle contraction in multicellular animals. |
| Signal | Molecules of proteins are embedded in the surface membrane of the cell, capable of changing their tertiary structure in response to the action of environmental factors, thus receiving signals from the external environment and transmitting commands to the cell. |
| Reserve | In the body of animals, proteins, as a rule, are not stored, with the exception of egg albumin, milk casein. But thanks to proteins in the body, some substances can be stored in reserve, for example, during the breakdown of hemoglobin, iron is not excreted from the body, but is stored, forming a complex with the ferritin protein. |
| Energy | With the breakdown of 1 g of protein to the final products, 17.6 kJ is released. First, proteins break down into amino acids, and then to the end products - water, carbon dioxide and ammonia. However, proteins are used as an energy source only when other sources (carbohydrates and fats) are used up. |
| catalytic | One of the most important functions of proteins. Provided with proteins - enzymes that accelerate the biochemical reactions that occur in cells. For example, ribulose biphosphate carboxylase catalyzes CO2 fixation during photosynthesis. |
Enzymes
Enzymes, or enzymes, is a special class of proteins that are biological catalysts. Thanks to enzymes, biochemical reactions proceed at a tremendous speed. The rate of enzymatic reactions is tens of thousands of times (and sometimes millions) higher than the rate of reactions involving inorganic catalysts. The substance on which an enzyme acts is called substrate.
Enzymes are globular proteins structural features Enzymes can be divided into two groups: simple and complex. simple enzymes are simple proteins, i.e. consist only of amino acids. Complex enzymes are complex proteins, i.e. in addition to the protein part, they include a group of non-protein nature - cofactor. For some enzymes, vitamins act as cofactors. In the enzyme molecule, a special part is isolated, called the active center. active center- a small section of the enzyme (from three to twelve amino acid residues), where the binding of the substrate or substrates occurs with the formation of an enzyme-substrate complex. Upon completion of the reaction, the enzyme-substrate complex decomposes into an enzyme and a reaction product(s). Some enzymes have (other than active) allosteric centers- sites to which regulators of the rate of enzyme work are attached ( allosteric enzymes).

Enzymatic catalysis reactions are characterized by: 1) high efficiency, 2) strict selectivity and direction of action, 3) substrate specificity, 4) fine and precise regulation. The substrate and reaction specificity of enzymatic catalysis reactions is explained by the hypotheses of E. Fischer (1890) and D. Koshland (1959).
E. Fisher (key-lock hypothesis) suggested that the spatial configurations of the active site of the enzyme and the substrate should correspond exactly to each other. The substrate is compared to the "key", the enzyme - to the "lock".
D. Koshland (hypothesis "hand-glove") suggested that the spatial correspondence between the structure of the substrate and the active center of the enzyme is created only at the moment of their interaction with each other. This hypothesis is also called induced fit hypothesis.
The rate of enzymatic reactions depends on: 1) temperature, 2) enzyme concentration, 3) substrate concentration, 4) pH. It should be emphasized that since enzymes are proteins, their activity is highest under physiologically normal conditions.
Most enzymes can only work at temperatures between 0 and 40°C. Within these limits, the reaction rate increases by about 2 times for every 10 °C rise in temperature. At temperatures above 40 °C, the protein undergoes denaturation and the activity of the enzyme decreases. At temperatures close to freezing, the enzymes are inactivated.
With an increase in the amount of substrate, the rate of the enzymatic reaction increases until the number of substrate molecules becomes equal to the number of enzyme molecules. With a further increase in the amount of substrate, the rate will not increase, since the active sites of the enzyme are saturated. An increase in the enzyme concentration leads to an increase in catalytic activity, since a larger number of substrate molecules undergo transformations per unit time.

For each enzyme, there is an optimal pH value at which it exhibits maximum activity (pepsin - 2.0, salivary amylase - 6.8, pancreatic lipase - 9.0). At higher or lower pH values, the activity of the enzyme decreases. With sharp shifts in pH, the enzyme denatures.
The speed of allosteric enzymes is regulated by substances that attach to allosteric centers. If these substances speed up the reaction, they are called activators if they slow down - inhibitors.
Enzyme classification
According to the type of catalyzed chemical transformations, enzymes are divided into 6 classes:
- oxidoreductase(transfer of hydrogen, oxygen or electron atoms from one substance to another - dehydrogenase),
- transferase(transfer of a methyl, acyl, phosphate or amino group from one substance to another - transaminase),
- hydrolases(hydrolysis reactions in which two products are formed from the substrate - amylase, lipase),
- lyases(non-hydrolytic addition to the substrate or the elimination of a group of atoms from it, while C-C, C-N, C-O, C-S bonds can be broken - decarboxylase),
- isomerase(intramolecular rearrangement - isomerase),
- ligases(connection of two molecules as a result of the formation C-C connections, C-N, C-O, C-S - synthetase).
Classes are in turn subdivided into subclasses and subsubclasses. In the current international classification, each enzyme has a specific code, consisting of four numbers separated by dots. The first number is the class, the second is the subclass, the third is the subclass, the fourth is the serial number of the enzyme in this subclass, for example, the arginase code is 3.5.3.1.
Go to lectures number 2"The structure and functions of carbohydrates and lipids"
Go to lectures №4"The structure and functions of ATP nucleic acids"
The chemical structure of proteins is represented by alpha-amino acids connected in a chain through a peptide bond. In living organisms, the composition determines the genetic code. In the synthesis process, in most cases, 20 amino acids of the standard type are used. Many of their combinations form protein molecules with a wide variety of properties. Amino acid residues often undergo post-translational modifications. They can occur before the protein begins to perform its functions, and in the process of its activity in the cell. In living organisms, several molecules often form complex complexes. An example is photosynthetic association.
Purpose of connections
Proteins are considered an important component of human and animal nutrition due to the fact that in their bodies all essential amino acids cannot be synthesized. Some of them should come with protein foods. The main sources of compounds are meat, nuts, milk, fish, grains. To a lesser extent, proteins are present in vegetables, mushrooms and berries. When digested by enzymes, consumed proteins are broken down into amino acids. They are already used in the biosynthesis of their own proteins in the body or are further decomposed - for energy.

History reference
The structure sequence of the insulin protein was determined for the first time by Frederick Senger. For his work, he received the Nobel Prize in 1958. Sanger used the sequencing method. Using X-ray diffraction, the three-dimensional structures of myoglobin and hemoglobin were subsequently obtained (at the end of the 1950s). The work was carried out by John Kendrew and Max Perutz.
Structure of a protein molecule
It includes linear polymers. They, in turn, consist of alpha-amino acid residues, which are monomers. In addition, the structure of the protein may include components having a non-amino acid nature and amino acid residues of a modified type. When designating components, 1- or 3-letter abbreviations are used. A compound containing from two to several tens of residues is often referred to as a "polypeptide". As a result of the interaction of the alpha-carboxyl group of one amino acid with the alpha-amino group of another, bonds appear (during the formation of the protein structure). In the compound, the C- and N- ends are isolated, depending on which group of the amino acid residue is free: -COOH or -NH 2. In the process of protein synthesis on the ribosome, as a rule, a methionine residue acts as the first terminal; the attachment of subsequent ones is carried out to the C-terminus of the previous ones.

Organization levels
They were proposed by Lindrem-Lang. Despite the fact that this division is considered somewhat obsolete, it is still used. It was proposed to allocate four levels of organization of connections. The primary structure of a protein molecule is determined genetic code and features of the gene. For more high levels characteristically formed during protein folding. The spatial structure of a protein is generally determined by the amino acid chain. However, it is quite flexible. It can be influenced by external factors. In this regard, it is more correct to speak about the conformation of the compound, which is the most favorable and energetically preferable.
1 level
It is represented by the sequence of amino acid residues of the polypeptide chain. As a rule, it is described using one or three letter designations. The primary structure of proteins is characterized by stable combinations of amino acid residues. They perform certain tasks. Such "conservative motives" remain preserved in the course of species evolution. They can often be used to predict the problem of an unknown protein. By evaluating the degree of similarity (homology) in amino acid chains from different organisms, one can determine the evolutionary distance formed between the taxa that make up these organisms. The primary structure of proteins is determined by sequencing or by the initial complex of its mRNA using the genetic code table.

Local ordering of a chain section
This is the next level of organization - the secondary structure of proteins. There are several types of it. The local ordering of the polypeptide chain region is stabilized by hydrogen bonds. The most popular types are:

Spatial structure
The tertiary structure of proteins includes elements of the previous level. They're stabilizing different types interactions. In this case, hydrophobic bonds are of paramount importance. Stabilization involves:
- covalent interactions.
- Ionic bonds that form between side amino acid groups that have opposite charges.
- Hydrogen interactions.
- hydrophobic bonds. In the process of interaction with the surrounding H 2 O elements, the protein is folded so that the side non-polar amino acid groups are isolated from the aqueous solution. Hydrophilic groups (polar) appear on the surface of the molecule.
The tertiary structure of proteins is determined by magnetic (nuclear) resonance, some types of microscopy, and other methods.
Laying principle
Studies have shown that between 2 and 3 levels it is convenient to single out another one. It is called "architecture", "laying motif". It is determined by the mutual arrangement of the components of the secondary structure (beta strands and alpha helices) within the boundaries of a compact globule - a protein domain. It can exist independently or be included in a larger protein along with other similar ones. It has been established that the styling motifs are rather conservative. They occur in proteins that have neither evolutionary nor functional relationships. The definition of architecture underlies rational (physical) classification.

Domain Organization
With the mutual arrangement of several chains of polypeptides in the composition of one protein complex, a quaternary structure of proteins is formed. The elements that make up its composition are formed separately on ribosomes. Only after the synthesis is completed, this protein structure begins to form. It may contain both different and identical polypeptide chains. The quaternary structure of proteins is stabilized by the same interactions as at the previous level. Some complexes may include several tens of proteins.
Protein structure: protective tasks
The polypeptides of the cytoskeleton, acting in some way as reinforcement, give many organelles a shape and participate in its change. Structural proteins provide protection to the body. An example of such a protein is collagen. It forms the basis in the intercellular substance of connective tissues. Keratin also has a protective function. It forms the basis of horns, feathers, hair and other derivatives of the epidermis. When toxins are bound by proteins, detoxification of the latter occurs in many cases. This is how the task of chemical protection of the body is performed. Particularly important in the process of neutralizing toxins in human body play liver enzymes. They are able to break down poisons or convert them into a soluble form. This contributes to faster transport of them from the body. Proteins present in the blood and other bodily fluids provide immune protection by eliciting a response to both attack by pathogens and injury. Immunoglobulins (antibodies and components of the complement system) are able to neutralize bacteria, foreign proteins and viruses.
Regulation mechanism
Protein molecules, which act neither as an energy source nor as a building material, control many intracellular processes. So, due to them, the regulation of translation, transcription, slicing, the activity of other polypeptides is carried out. The mechanism of regulation is based on enzymatic activity or manifests itself through specific binding to other molecules. For example, transcription factors, activator polypeptides, and repressor proteins can control the rate of gene transcription. At the same time, they interact with the regulatory sequences of genes. Protein phosphatases and protein kinases play the most important role in controlling the course of intracellular processes. These enzymes start or suppress the activity of other proteins by adding or removing phosphate groups from them.

Signal task
It is often combined with a regulatory function. This is because many intracellular as well as extracellular polypeptides can transmit signals. Growth factors, cytokines, hormones and other compounds have this ability. Steroids are transported through the blood. The interaction of the hormone with the receptor acts as a signal, due to which the response of the cell is triggered. Steroids control the content of compounds in the blood and cells, reproduction, growth and other processes. An example is insulin. It regulates glucose levels. The interaction of cells is carried out by means of signal protein compounds transmitted through the intercellular substance.
Element transport
Soluble proteins involved in the movement of small molecules have a high affinity for the substrate present in high concentration. They also have the ability to easily release it in areas with a low content. An example is the transport protein hemoglobin. It moves oxygen from the lungs to other tissues, and from them it transfers carbon dioxide. Some membrane proteins are also involved in the transport of small molecules through the cell walls, changing them. The lipid layer of the cytoplasm is water resistant. This prevents the diffusion of charged or polar molecules. Membrane transport connections are usually divided into carriers and channels.
Backup connections
These proteins form the so-called reserves. They accumulate, for example, in plant seeds, animal eggs. Such proteins act as a reserve source of matter and energy. Some compounds are used by the body as an amino acid reservoir. They, in turn, are the precursors of active substances involved in the regulation of metabolism.
Cell receptors
Such proteins can be located both directly in the cytoplasm and embedded in the wall. One part of the connection receives a signal. As a rule, it is a chemical substance, and in some cases - a mechanical effect (stretching, for example), light and other stimuli. In the process of signal exposure to a certain fragment of the molecule - the receptor polypeptide - its conformational changes begin. They provoke a change in the conformation of the rest of the cell, which carries out the transmission of the stimulus to other components of the cell. Sending a signal can be done in different ways. Some receptors are able to catalyze chemical reaction, the latter act as ion channels that close or open under the influence of a stimulus. Some compounds specifically bind intermediary molecules within the cell.
Motor polypeptides
There is a whole class of proteins that provide the movement of the body. Motor proteins are involved in muscle contraction, cell movement, activity of flagella and cilia. Due to them, directed and active transport is also performed. Kinesins and dyneins carry out the transfer of molecules along the microtubules using ATP hydrolysis as an energy source. The latter move organelles and other elements towards the centrosome from peripheral cellular regions. Kinesins move in the opposite direction. Dyneins are also responsible for the activity of flagella and cilia.
- In contact with 0
- Google Plus 0
- OK 0
- Facebook 0