All living things on the planet are made up of numerous cells. They maintain the orderliness of their organization with the help of genetic information contained in the nucleus, which is stored, transmitted and implemented. high-molecular complex compounds- Nucleic acids. These acids, in turn, consist of monomer units - nucleotides.
In contact with
The role of nucleic acids cannot be overestimated. The normal vital activity of an organism is determined by the stability of their structure. If any deviations occur in the structure, the quantity or sequence changes - this necessarily leads to changes in the cellular organization. changes in the activity of physiological processes and vital activity of cells.
The concept of a nucleotide
 Like squirrels , nucleic acids are essential for life. It is the genetic material of all living organisms, including viruses.
Like squirrels , nucleic acids are essential for life. It is the genetic material of all living organisms, including viruses.
Elucidation of the structure of one of the two types of DNA nucleic acids made it possible to understand how information is stored in living organisms that is necessary for regulating life and how it is transmitted to offspring. A nucleotide is a monomeric unit that forms more complex compounds - nucleic acids. Storage is impossible without them., reproduction and transmission of genetic information. Free nucleotides are the main components that are involved in energy and signaling processes. They support the normal functioning of individual cells and the organism as a whole. Long molecules, polynucleotides, are built from them. To understand the structure of a polynucleotide, one should understand the structure of nucleotides.
What is a nucleotide? DNA molecules are assembled from small monomeric compounds. In other words, a nucleotide is an organic complex compound that is an integral part of nucleic acids and other biological compounds necessary for the life of a cell.
Composition and basic properties of nucleotides
 The composition of a nucleotide molecule (mononucleotide) in a certain sequence includes three chemical compounds:
The composition of a nucleotide molecule (mononucleotide) in a certain sequence includes three chemical compounds:
- Pentose or Pentagonal Sugar:
- deoxyribose. These nucleotides are called deoxyribonucleotides. They are part of the DNA;
- ribose. Nucleotides are part of RNA and are called ribonucleotides.
2. A nitrogenous pyrimidine or purine base linked to a carbon atom of a sugar. This compound is called a nucleoside
3. Phosphate group consisting of residues phosphoric acid(from one to three). Attached to the sugar carbon by ester bonds forming a nucleotide molecule.
The properties of nucleotides are:
- participation in metabolism and other physiological processes that occur in the cell;
- exercise control over reproduction and growth;
- storage of information about inherited traits and protein structure.
Nucleic acids
 Sugar in nucleic acids is represented by pentose. In RNA, the five-carbon sugar is called ribose; in DNA, it is called deoxyribose. Each pentose molecule has five carbon atoms, of which four form a ring with an oxygen atom, and the fifth atom is part of the HO-CH2 group.
Sugar in nucleic acids is represented by pentose. In RNA, the five-carbon sugar is called ribose; in DNA, it is called deoxyribose. Each pentose molecule has five carbon atoms, of which four form a ring with an oxygen atom, and the fifth atom is part of the HO-CH2 group.
In a molecule the position of the carbon atom indicated by a number with a prime (for example: 1C´, 3C´, 5C´). Since all the processes of reading from a nucleic acid molecule hereditary information there is a strict directionality, the numbering of carbon atoms and their location serve as an indicator of the right direction.
A nitrogenous base is attached to the first carbon atom 1C´ in the sugar molecule.
The third and fifth carbon atoms of the hydroxyl group (3C´, 5C´) are joined by a phosphoric acid residue, which determines the chemical belonging to the acid group of DNA and RNA.
Composition of nitrogenous bases
 Types of nucleotides according to the nitrogenous base of DNA:
Types of nucleotides according to the nitrogenous base of DNA:
The first two classes are purines:
- adenine (A);
- guanine (G).
The last two belong to the class of pyrimidines:
- thymine (T);
- cytosine (C).
Purine compounds by molecular weight heavier than pyrimidines.
RNA nucleotides by nitrogenous compound are represented by:
- guanine;
- adenine;
- uracitol;
- cytosine.
Just like thymine, uracil is a pyrimidine base. Often in the scientific literature, nitrogenous bases are denoted by Latin letters (A, T, C, G, U).
Pyrimidines, namely thymine, cytosine, uracil, are represented by a six-membered ring consisting of two nitrogen atoms and four carbon atoms, sequentially numbered from 1 to 6.
The purines (guanine and adnin) are composed of imidazole and pyrimidine. Purine bases have four nitrogen atoms and five carbon atoms. Each atom has its own number from 1 to 9.
The result of the combination of nitrogenous residues with pentose residues is a nucleoside. Nucleotide is the combination of a phosphate group with a nucleoside.
Formation of phosphodiester bonds
 It is necessary to understand the question of how nucleotides are connected into a polypeptide chain, how many of them are involved in the process, forming a nucleic acid molecule due to phosphodiester bonds.
It is necessary to understand the question of how nucleotides are connected into a polypeptide chain, how many of them are involved in the process, forming a nucleic acid molecule due to phosphodiester bonds.
When two nucleotides interact, a dinucleotide is formed. A new compound is formed by condensation, when a phosphodiester bond occurs between the hydroxy group of the pentose of one monomer and the phosphate residue of the other.
The synthesis of a polynucleotide is the multiple repetition of this reaction. The assembly of polynucleotides is a complex process that ensures the growth of the chain from one end.
 DNA molecules, like protein molecules, have primary, secondary and tertiary structures. The primary structure in a DNA chain is determined by the sequence of nucleotides. The secondary structure is based on the formation of hydrogen bonds. At DNA double helix synthesis there is a certain pattern and sequence: the thymine of one chain corresponds to the adenine of the other; cytosine - guanine, and vice versa. Nucleide compounds create a strong bond of chains, with an equal distance between them.
DNA molecules, like protein molecules, have primary, secondary and tertiary structures. The primary structure in a DNA chain is determined by the sequence of nucleotides. The secondary structure is based on the formation of hydrogen bonds. At DNA double helix synthesis there is a certain pattern and sequence: the thymine of one chain corresponds to the adenine of the other; cytosine - guanine, and vice versa. Nucleide compounds create a strong bond of chains, with an equal distance between them.
Knowing the nucleotide sequence one strand of DNA is possible according to the principle of addition or complementarity to complete the second.
The tertiary structure of DNA is formed by three-dimensional complex connections. This makes the molecule more compact so that it can fit freely in the small volume of the cell. the length of E. coli DNA is more than 1 mm, while the length of the cell itself is less than 5 microns.
The number of pyrimidine bases is always equal to the number of purines. The distance between nucleotides is 0.34 nm. It is a constant, just like molecular weight.
Functions and properties of DNA
 The main functions of DNA:
The main functions of DNA:
- preserves hereditary information;
- transmission (doubling/replication);
- transcription, implementation;
- DNA autoreproduction. Functioning of the replicon.
The process of self-reproduction of a nucleic acid molecule is accompanied by the transfer of copies of genetic information from cell to cell. For its implementation, a set of specific enzymes is required. In this semi-conservative type process, a replication fork is formed.
A replicon is a unit of the replication process of a region of the genome controlled by a single replication initiation point. Typically, the prokaryotic genome is a replicon. Replication from the point of initiation goes both ways, sometimes at different speeds.
RNA molecule - structure
 RNA is a single polynucleotide chain that is formed through covalent bonds between a phosphate residue and a pentose. It is shorter than DNA, has a different sequence, and differs in the species composition of nitrogenous compounds. Thymine pyrimidine base in RNA replaced by uracil.
RNA is a single polynucleotide chain that is formed through covalent bonds between a phosphate residue and a pentose. It is shorter than DNA, has a different sequence, and differs in the species composition of nitrogenous compounds. Thymine pyrimidine base in RNA replaced by uracil.
RNA can be of three types, depending on the functions that are performed in the body:
- information (mRNA) - very diverse in nucleotide composition. It is a kind of matrix for the synthesis of a protein molecule, transfers genetic information to ribosomes from DNA;
- transport (tRNA) on average consists of 75-95 nucleotides. She endures essential amino acid in the ribosome to the site of polypeptide synthesis. Each type of tRNA has its own, unique sequence of nucleotides or monomers;
- ribosomal (rRNA) usually contains from 3000 to 5000 nucleotides. The ribosome is a necessary structural component involved in critical process occurring in the cell - protein biosynthesis.
The role of the nucleotide in the body
In the cell, nucleotides perform important functions:
- are bioregulators;
- used as building blocks for nucleic acids;
- are part of the main source of energy in the cell - ATP;
- participate in numerous metabolic processes in cells;
- are carriers of reducing equivalents in cells (FAD, NADP+; NAD+; FMN);
- can be considered as messengers of regular extracellular synthesis (cGMP, cAMP).
Free nucleotides are the main components that are involved in energy and signaling processes. They support the normal functioning of individual cells and the body as a whole.
Nucleotide– nucleoside + one or more phosphoric acid residues. Nucleoside- nitrogenous base and pentose molecule. The composition of nucleotides includes two purine bases (adenine and guanine) and 3 pyrimidine bases (thymine, uracil, cytosine). Sometimes there are minor nitrogenous bases: pseudouracil, methyluridine, methylcytosine, methyladenine.
Nomenclature:
Primary structure of NK- a polynucleotide chain with a strictly defined sequence of nucleotides interconnected by a 3'-5'-phosphodiester bond.
Properties of nucleotides: 1) acquire negative charge 2) possess brightly
Pronounced acidic properties.
Features of the structure, function and distribution of DNA and RNA in the cell:
|
Localized mainly in the nucleus, also in mitochondria and chloroplasts |
Located mainly in the cytoplasm |
|
The structure includes A, T, G, C + deoxyribose + phosphoric acid residue. |
The structure includes A, U, G, C + ribose + phosphoric acid residue |
|
Double helix (6 types are known: A-E, Z, the predominant B-form) |
Single-stranded (although it can fold to form "hairpins"). Has varieties (mRNA, mRNA, tRNA) |
|
Vary in size (DNA usually consists of a large number of nucleotides) |
|
|
1. Provides Protein Synthesis 2. Carrier of hereditary information |
Provide protein synthesis |
|
Obeys the rules of Chargaff |
Doesn't obey Chargaff's rules |
DNA primary structure analysis method (Sanger):
Based on the DNA polymerase reaction: isolation of DNA ® cutting it with restriction enzymes ® denaturation of DNA fragments and obtaining single-stranded molecules used as a template ® add a primer and substrates for DNA synthesis ® divide the mixture into four test tubes, add one of the stop nucleotides to each ( dideoxynucleotides) and DNA polymerase ® synthesis stops when DNA polymerase encounters a stop nucleotide ® after the end, in each tube there are fragments ending in a certain nucleotide ® fragments are separated by electrophoresis in agarose gel and analyzed.
NUCLEOTIDES NUCLEOTIDES
nucleoside phosphates, phosphate esters of nucleosides. They consist of a nitrogenous base (usually purine or pyrimidine), a ribose carbohydrate (ribonucleotides) or deoxyribose (deoxyribonucleotides) and one or more. residues of phosphoric acid. Compounds from two residues N. called. dinucleotides, from several - oligonucleotides, from many - polynucleotides. N. are a part of nucleinic to - t (polynucleotides), the most important coenzymes (NAD, NADP, FAD, CoA) and other biologically active connections. Free N. in the form of nucleoside mono-, di- and triphosphates, which means that the quantities are contained in living cells. Nucleoside triphosphates - N., containing 3 residues of phosphoric acid, are energy-rich (macroergic) compounds, sources and carriers of chemical. phosphate bond energy. ATP plays a special role - a universal energy accumulator that provides decomp. life processes. High energy. phosphate bonds of nucleoside triphosphates are used in the synthesis of polysaccharides (uridine triphosphate, ATP), proteins (GTP, ATP), lipids (cytidine triphosphate, ATP). Nucleoside triphosphates are also substrates for the synthesis of nucleic acids. Uridine diphosphate is involved in carbohydrate metabolism as a carrier of monosaccharide residues, cytidine diphosphate (carrier of choline and ethanolamine residues) in lipid metabolism. Cyclic nucleotides play an important regulatory role in the body. Free nucleoside monophosphates are formed by synthesis (see PURINE BASES, PYRIMIDINE BASES) or by hydrolysis of nucleic acids under the action of nucleases. Sequential phosphorylation of nucleoside monophosphates leads to the formation of the corresponding nucleoside di- and nucleoside triphosphates. N.'s disintegration occurs under the action of nucleotidase (in this case, nucleosides are formed), as well as nucleotide pyrophosphorylases, which catalyze the reversible reaction of N.'s cleavage to free bases and phosphoribosyl pyrophosphate. (See ADENOSINPHOSPHORIC ACIDS, GUANOSINPHOSPHORIC ACIDS, INOSINPHOSPHORIC ACIDS, THYMIDINPHOSPHORIC ACIDS, CYTIDINPHOSPHORIC ACIDS, URIDINPHOSPHORIC ACIDS).
.(Source: Biological encyclopedic Dictionary." Ch. ed. M. S. Gilyarov; Editorial: A. A. Babaev, G. G. Vinberg, G. A. Zavarzin and others - 2nd ed., corrected. - M.: Sov. Encyclopedia, 1986.)
nucleotidesNatural compounds from which, like links, chains are built nucleic acids; are also part of the most important coenzymes (organic compounds of non-protein nature - a component of some enzymes) and other biologically active substances, serve as energy carriers in cells.
The molecule of each nucleotide (mononucleotide) consists of three chemically different parts. Firstly, it is a five-carbon sugar (pentose) - ribose (in this case, nucleotides are called ribonucleotides and are part of ribonucleic acids, or RNA) or deoxyribose (nucleotides are called deoxyribonucleotides and are part of deoxyribonucleic acids, or DNA). Secondly, it is a purine or pyrimidine nitrogenous base. When bonded to the carbon atom of the sugar, it forms a compound called a nucleoside. And finally, one, two or three phosphoric acid residues attached by ester bonds to the sugar carbon form a nucleotide molecule. The nitrogenous bases of DNA nucleotides are the purines adenine and guanine and the pyrimidines cytosine and thymine. RNA nucleotides contain the same bases as DNA, but thymine in them is replaced by uracil, which is similar in chemical structure.
Nitrogenous bases and, accordingly, the nucleotides that include them in the biological literature are usually denoted by the initial letters (Latin or Russian) of their names: adenine - A (A), guanine - G (G), cytosine - C (C), thymine - T (T ), uracil - U(U). The connection of two nucleotides is called a dinucleotide, several - an olinonucleotide, many - a polynucleotide, or nucleic acid.
In addition to the fact that nucleotides form chains of DNA and RNA, they are coenzymes, and nucleotides bearing three phosphoric acid residues (nucleoside triphosphates) are sources of chemical energy contained in phosphate bonds. The role of such a universal carrier of energy as adenosine triphosphate(ATP).
A special group is made up of cyclic nucleotides that mediate the action of hormones in the regulation of metabolism in cells.
.(Source: "Biology. Modern Illustrated Encyclopedia." Editor-in-Chief A.P. Gorkin; M.: Rosmen, 2006.)
See what "NUCLEOTIDES" are in other dictionaries:
- (nucleoside phosphates) phosphate esters of nucleosides; They consist of a nitrogenous base (purine or pyrimidine), a carbohydrate (ribose or deoxyribose), and one or more phosphoric acid residues. Connections from one, two, three, several ... ... Big Encyclopedic Dictionary
nucleotides- ov, pl. nucleotides nucleus. biol. organic matter component nucleic acids and coenzymes of many enzymes. N. play important role in animal metabolism and flora. Krysin 1998. Lex. SIS 1964: Nucleotides/dyes… Historical dictionary gallicisms of the Russian language
nucleotides- - Nucleoside esters with phosphoric acid ... Concise Dictionary biochemical terms
Nucleotides, phosphate esters of nucleosides, nucleoside phosphates. Free nucleotides, in particular ATP, cAMP, ADP, play an important role in energy and informational intracellular processes, and are also constituent parts of nucleic ... ... Wikipedia
Nucleoside phosphates, compounds that make up Nucleic acids, many coenzymes, and other biologically active compounds; each N. is built from a nitrogenous base (usually purine or pyrimidine), a carbohydrate (ribose or ... ... Great Soviet Encyclopedia
- (nucleoside phosphates), phosphate esters of nucleosides; They consist of a nitrogenous base (purine or pyrimidine), a carbohydrate (ribose or deoxyribose), and one or more phosphoric acid residues. Connections from one, two, three, several ... encyclopedic Dictionary
Nucleotides- Model of adenine molecule. NUCLEOTIDES, organic compounds consisting of a nitrogenous base (adenine, guanine, cytosine, thymine, uracil), a carbohydrate (ribose or deoxyribose) and one or more phosphoric acid residues. Nucleotides - ... ... Illustrated Encyclopedic Dictionary
- (lat. nucleus nucleus) organic matter, consisting of a purine or pyrimidine base, a carbohydrate and phosphoric acid; an integral part of nucleic acids and coenzymes of many enzymes; a number of nucleotides (adenylic acid, adenosindi and ... ... Dictionary of foreign words of the Russian language
Nucleotides- molecules consisting of five nitrogenous bases (cytosine, uracil, thymine, adenine and guanine), ribose (or deoxyribose) and a phosphoric acid residue. Nucleotides can join together to form polynucleotides (nucleic acids) ... Concepts modern natural science. Glossary of basic terms
- (nucleoside phosphates), esters of phosphoric acid and nucleosides one or more. hydroxylam of a monosaccharide residue; in a broader sense Comm., in which the monosaccharide residue of the nucleoside or its non-natural analogue is esterified with one or several. mono… … Chemical Encyclopedia
Books
- Biologically active substances in physiological and biochemical processes in the animal body, M. I. Klopov, V. I. Maksimov. The manual outlines modern ideas about the structure, mechanism of action, role in the life processes and functions of the body of biologically active substances (vitamins, enzymes, ...
Nucleotides are phosphate esters of nucleosides.
Their chemical composition: nitrogenous base (A.O.) + pentose + phosphoric acid
Phosphoric esters are formed with the participation of hydroxyl groups of pentoses. The positions of the phosphoric ester groups are usually denoted using the designation ("), for example: 5 ", 3 "
Preliminary brief information: nucleotides play an extremely important role in the life of the cell.
Classification of nucleotides
Nucleotides made up of one molecule A.O, pentose, phosphoric acid, called mononucleotides. Mononucleotides can contain one phosphoric acid molecule, two or three phosphoric acid molecules connected to each other.
Combination of two mononucleotides called dinucleotide. AT The composition of a dinucleotide usually contains various nitrogenous bases or one other cyclic compound, for example, a vitamin ..
Cyclic mononucleotides play a special role in biochemical processes.
Nomenclature of mononucleotides.
Go to title nucleoside added based on the amount of phosphate residues, ʼʼ monophosphateʼʼ, ʼʼ diphosphateʼʼ, ʼʼ triphosphateʼʼ, indicating their position in the pentose cycle - digital designation of the place with a sign ("),
The position of the phosphate group in position (5") is the most common and typical, and therefore it can be omitted (AMP, GTP, UTP, d AMF, etc.)
The remaining positions are indicated necessarily (3 "- AMF, 2" - AMF, 3 "- d AMF)
5"-adenosine monophosphate
(5"- AMF or AMF)
Names of the most common nucleotides
| nucleoside | nucleoside monophosphate | nucleoside diphosphate | nucleoside triphosphate |
| adenosine | 5 "-Adenosine monophosphate (5" - AMP or AMP) 5 "-adenylic acid | 5 "-adenosine diphosphate (5"-ADP or ADP) | 5 "-adenosine triphosphate (5"-ATP or ATP) |
| adenosine | 3"-adenosine monophosphate (3"-AMP) 3"-adenylic acid | not found in vivo | not found in vivo |
| guanosine | 5 "-guanosine monophosphate (5" - GMF or GMF) | 5 "-guanosine diphosphate (5" - HDF or HDF) | 5 "-guanosine triphosphate (5" - GTP or GTP) |
| guanosine | 3"-guanosine monophosphate (3"- GMP) 3"-guanilic acid | not found in vivo | not found in vivo |
| deoxy adenosine | 5 "-deoxyadenosine monophosphate (5"- d AMF or d AMF) | 5 "-deoxyadenosine diphosphate (5"- d ADFili d ADP) | 5 "-deoxyadenosine triphosphate (5"- d ATFili d ATP) |
| uridine | 5 "-uridine monophosphate (5" - UMF or UMF) | 5 "-uridine diphosphate (5" - UDP or UDP) | 5 "-uridine triphosphate (5" - UTP or UTP) |
| cytidine | 5 "-cytidine monophosphate (5" - CMF or CMF) | 5 "-cytidine diphosphate (5" - CDP or CDP) | 5 "-cytidine triphosphate (5" - CTP or CTP) |
Nucleotides formed with the participation of ribose can contain phosphoric acid residues in three positions (5", 3", 2"), and with the participation of deoxyribose - only in two positions (5", 3"), in position 2" there is no hydroxy group. This circumstance is very important for the structure of DNA.
The absence of a hydroxy group in the second position has two important consequences:
The polarization of the glycosidic bond in DNA decreases and it becomes more resistant to hydrolysis.
2-O-deoxyribose cannot undergo either epimerization or conversion to ketosis.
In the cell, nucleoside monophosphate is sequentially converted to diphosphate, and then to triphosphate.
For example: AMP ---> ADP ---> ATP
The biological role of nucleotides
All nucleoside diphosphates and nucleoside triphosphates belong to high-energy (macroergic) compounds.
Nucleoside triphosphates participate in the synthesis of nucleic acids, provide the activation of bioorganic compounds and biochemical processes that take place with the expenditure of energy. Adenosine triphosphate (ATP) is the most abundant macroergic compound in the human body. The content of ATP in the skeletal muscles of mammals is up to 4 g / kg, the total content is about 125 ᴦ. In humans, the rate of ATP metabolism reaches 50 kg/day. Hydrolysis of ATP produces adenosine diphosphate(ADP)
macroergic connections
ATP contains different types chemical bonds:
N-β- glycosidic
Ester
Two anhydride (biologically macroergic)
In conditions in vivo hydrolysis of the macroergic bond of ATP is accompanied by the release of energy (about 35 kJ / mol), which provides other energy-dependent biochemical processes.
ATP + H2O - enzyme ATP hydrolase --> ADP + H3 PO4
In aqueous solutions ADP and ATP unstable . At 0 0 SATP is stable in water for only a few hours, and when boiled for 10 minutes.
Under the action of alkali, two terminal phosphates (anhydride bonds) are easily hydrolyzed, and the last one (ester bond) is difficult. During acid hydrolysis, the N-glycosidic bond is easily destroyed.
For the first time, ATP was isolated from muscles in 1929 ᴦ. K. Loman. Chemical synthesis carried out in 1948 ᴦ. A. Todd.
Cyclic nucleotides are mediators in the transmission of hormone signals by changing the activity of enzymes in the cell.
Οʜᴎ are formed from nucleoside triphosphates.
ATP - cyclase enzyme --> cAMP + H4 P2 O7
After the action is completed, hydrolysis of the cyclic nucleotide occurs. . Two compounds can be formed 5 "-AMP and 3" -AMP, but under biological conditions only 5 "-AMP is formed,
Cyclic adenosine monophosphate (cAMP)
11.5. Structure of nucleic acids
The primary structure of RNA and DNA is the sequential connection of nucleotides in a polynucleotide chain. The skeleton of a polynucleotide chain consists of carbohydrate and phosphate residues, heterocyclic nitrogenous bases are connected to carbohydrates through an N-β - glycosidic bond. From a biological point of view, triplets - blocks of nucleotides from three nitrogenous bases, each of which encodes an amino acid or has a certain signal function, are of the utmost importance.
The structure of the NC can be represented schematically:
5" 3" 5" 3" 5" 3"
phosphate -- pentose -- phosphate -- pentose -- phosphate -- pentose-OH
AT primary structure DNA Start chains are determined by the pentose containing phosphate in position 5. Pentoses in the polynucleotide chain are connected via phosphate bonds 3 "→ 5". On the end chains in position 3 "- pentoses OH group remains free.
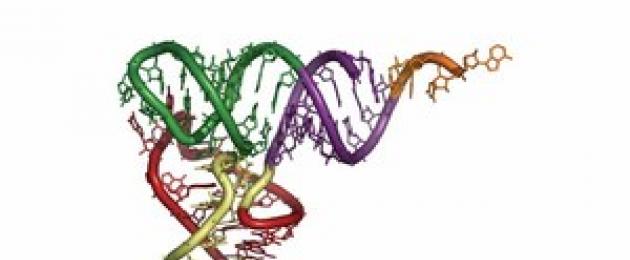
Higher order DNA structure - double helix
Scientific Description secondary structure of DNA refers to the greatest discoveries humanity in the twentieth century. Biochemist D. Watson and physicist F. Creek in 1953 proposed a model of the structure of DNA and the mechanism of the replication process. In 1962 ᴦ. they were awarded the Nobel Prize.
In a popular form, the story is described in James Watson's book ʼʼ The Double Helixʼʼ, M.: Mir, 1973. The book tells the story in a very interesting way. joint work, with humor and light irony of the author to such a significant event, the happy ʼʼculpritsʼʼ of which were two young scientists. Since the discovery of the structure of DNA, mankind has received a tool for the development of a new direction - biotechnology, protein synthesis by gene recombination (hormones in the medical industry receive insulin, erythropoietin, and many others).
Research contributed to the discovery of the structure of DNA E.Chargaff in a relationship chemical composition DNA. He found out:
The number of pyrimidine bases is equal to the number of purines
The amount of thymine is equal to the amount of adenine, and the amount of cytosine is equal to the amount
A = T G = C
A + G = T + C
A + C = T + G
These relationships are called Chargaff rules .
The DNA molecule consists of two twisted helices. The skeleton of each helix is a chain of alternating residues of deoxyribose and phosphoric acid. The spirals are oriented in such a way that they form two unequal spiral grooves that run parallel to the main axis. These grooves are filled with proteins histones. Nitrogenous bases are located inside the helix, almost perpendicular to the main axis and form complementary pairs between the chains A…T and G…C.
The total length of DNA molecules in each cell reaches 3 cm. The average cell diameter is 10–5 m, the DNA diameter is only 2‣‣‣10–9 m.
The main parameters of the double helix:
* diameter 1.8 - 2nm,
* on one turn 10 nucleotides
* coil pitch height ~ 3.4 nm
* distance between two nucleotides 0.34 nm.
The bases are located perpendicular to the axis of the chain.
* direction of polynucleotide chains antiparallel
* communication between furanose cycles of deoxyribose through
phosphoric acid is carried from position 3` to position 5` in
each of the chains.
* The beginning of the chain - the hydroxyl group of the pentose is phosphorylated in the position
5`, the end of the chain is the free hydroxyl group of pentose in position 3`.
* In the composition of DNA and RNA, the nucleoside fragments are in the anti-conformation; the pyrimidine ring of purine is located to the right of the glycosidic bond. Only this position allows the formation of a complementary pair (see nucleotide formulas)
* There are three types of interactions between nitrogenous bases:
1. “Transverse”, complementary pairs of two chains are involved. A ʼʼcyclicʼʼ electron transfer occurs between two nitrogenous bases (T - A, U - C), an additional p-electron system is formed, which provides additional interaction and protects nitrogenous bases from undesirable chemical influences. Between adenine and thymine make two hydrogen bonds, and three hydrogen bonds between guanine and cytosine.
2. ʼʼ Vertical ʼʼ (stacking), due to stacking in “stacks”, nitrogenous bases of one chain are involved. ʼʼStacking interactionʼʼ has even more value in stabilizing the structure than the interaction in complementary pairs
3. Interaction with water plays an essential role in maintaining the spatial structure of the double helix, which adopts the most compact structure to reduce the surface of contact with water and directs hydrophobic heterocyclic bases into the interior of the helix.
Structure and composition of nucleoprotein complexes
Several types of interactions are involved in the binding of a nucleic acid to a protein:
electrostatic
Hydrogen bonds
hydrophobic
Real three-dimensional models of DNA, ribosomes, informosomes, and nucleic acids of viruses were built based on the results of X-ray diffraction analysis using computer simulation.
Histone proteins of DNA have pronounced basic properties and differ a high degree evolutionary conservatism. According to the ratio of two basic amino acids lysine / arginine, they are divided into 5 classes: H1, H2A, H 2B, H3, H4
Nucleotides - concept and types. Classification and features of the category "Nucleotides" 2017, 2018.
Along with amino acids, the most important group of nitrogenous substances are nucleotides. Them biological significance for the life of organisms is determined by the fact that they are used to build molecules of nucleic acids - deoxyribonucleic (DNA) and ribonucleic (RNA), are part of the catalytic centers of enzymes, participate in bioenergetic processes and the synthesis of carbohydrates, lipids, proteins, alkaloids and other substances . Some nucleotides are capable of performing regulatory functions.
Main structural components nucleotides - nitrogenous bases, pentoses (ribose or deoxyribose) and a residue of orthophosphoric acid. Depending on the carbohydrate component, two groups of nucleotides are distinguished: ribonucleotides containing a ribose residue and deoxyribonucleotides containing a deoxyribose residue. Deoxyribonucleotides are used by organisms for DNA synthesis, and ribonucleotides are part of RNA, enzymes, and macroergic nucleoside polyphosphates.
Ribose and deoxyribose in the composition of nucleotides are in the b-D-furanose form:
Nucleotides are formed from two types of nitrogenous bases - derivatives of pyrimidine and purine. They exhibit the properties of bases in an aqueous solution when interacting with water molecules. Of the pyrimidine bases, uracil, thymine and cytosine are the most important as the main structural units of the nucleotides that form nucleic acids. In addition to them, other bases are also known - 5-methylcytosine, pseudouracil, 5-hydroxymethylcytosine, etc. 5-methylcytosine and 5-hydroxymethylcytosine in a small amount can
Of the purine bases, adenine and guanine are of the greatest importance, since they are used for the synthesis of nucleic acids. In the composition of nucleic acids, other bases were also found in a small amount, which are formed as a result of the chemical modification of adenine and guanine: 7-methylguanine, 2-methyladenine, N-dimethylguanine, etc. Important intermediate metabolites are hypoxanthine, xanthine, allantoin. In some plants, they can accumulate in a free state.
All nitrogenous bases intensely absorb ultraviolet light at wavelengths of 200-280 nm.
When nitrogenous bases are combined with a ribose or deoxyribose molecule, compounds are formed called nucleosides, since a glycosidic bond arises between the pentose and the base. The bases in this case can be considered as aglycons in relation to pentose.
In nucleosides, a glycosidic bond occurs between the first carbon atom of the pentose in the b-furanose form and the nitrogen of the purine (in the ninth position) or pyrimidine (in the first position) base. The nitrogenous bases adenine, guanine, cytosine and uracil form, when combined with ribose, nucleosides - adenosine, guanosine, cytidine and uridine,
and with deoxyribose - deoxyadenosine, deoxyguanosine, deoxycytidine, deoxyuridine. Thymine combines with deoxyribose to form deoxythymidine.
Nitrogenous bases and nucleosides can be accumulated in plants in significant amounts due to the intense degradation of nucleic acids.
Nucleoside phosphate esters are called nucleotides. In the composition of nucleotides, orthophosphoric acid residues can be attached to the fifth or third carbon atom of ribose or deoxyribose, and in some ribonucleotides also to the second carbon atom of ribose. In free nucleotides, the phosphate group is usually located at the fifth carbon atom of ribose or deoxyribose. In a neutral medium, phosphoric acid residues in nucleotide molecules are strongly dissociated, as a result of which cations can be attached, therefore, during chemical isolation, nucleotides crystallize in the form of salts.
The study of the spatial structure of nitrogenous bases by X-ray diffraction analysis shows that they all have an almost planar conformation. They quite easily rearrange double bonds, which is accompanied by tautomeric transformations. For example, guanine can exist in two tautomeric forms:
The plane of the heterocyclic core of the base in the structure of nucleosides and nucleotides can occupy two positions in space with respect to the pentose, forming two opposite conformations - syn-conformation and anti-conformation. AT anti-conformation, the structure of the nitrogenous base is deployed from pentose, and in syn-conformation is oriented above its plane. In the free state, pyrimidine nucleotides are found predominantly in anti-conformations, and purines quite easily pass from one form to another.
Due to the fact that nucleotides have strongly pronounced acidic properties, they are called acids, taking into account the names of the nitrogenous bases and the carbohydrate component. So, for example, a ribonucleotide having an adenine residue is called adenylic acid, or adenosine monophosphate (AMP). Deoxyribonucleotide derived from thymine is called deoxythymidylic acid, or deoxythymidine monophosphate (dTMP). The names of other nucleotides are presented in Table 2.
In plants, cyclic forms of nucleotides, adenosine monophosphate and guanosine monophosphate, have been found, which apparently perform regulatory functions. The structure of cyclic AMP can be represented by the following formula:
2 . Names of the most important nucleotides.
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0