Properties of water and its role in the cell:
In the first place among the substances of the cell is water. It makes up about 80% of the mass of the cell. Water is doubly important for living organisms, because it is necessary not only as a component of cells, but for many and as a habitat.
1. Water determines physical properties cells - its volume, elasticity.
2. Many chemical processes take place only in an aqueous solution.
3. Water is a good solvent: many substances enter the cell from the external environment in an aqueous solution, and in an aqueous solution, waste products are removed from the cell.
4. Water has a high heat capacity and thermal conductivity.
5. Water has a unique property: when it is cooled from +4 to 0 degrees, it expands. Therefore, ice is lighter than liquid water and remains on its surface. This is very important for organisms living in the aquatic environment.
6. Water can be a good lubricant.
The biological role of water is determined by the small size of its molecules, their polarity and the ability to combine with each other by hydrogen bonds.
Biological functions of water:
transport. Water ensures the movement of substances in the cell and body, the absorption of substances and the excretion of metabolic products. In nature, water carries waste products to soils and water bodies.
metabolic. Water is the medium for all bio chemical reactions, an electron donor in photosynthesis; it is necessary for the hydrolysis of macromolecules to their monomers.
water is involved in the formation of lubricating fluids and mucus, secrets and juices in the body.
With very few exceptions (bone and tooth enamel), water is the predominant component of the cell. Water is necessary for the metabolism (exchange) of the cell, since physiological processes occur exclusively in the aquatic environment. Water molecules are involved in many enzymatic reactions of the cell. For example, the breakdown of proteins, carbohydrates and other substances occurs as a result of their interaction with water catalyzed by enzymes. Such reactions are called hydrolysis reactions.
Water serves as a source of hydrogen ions during photosynthesis. Water in the cell is in two forms: free and bound. Free water makes up 95% of all water in the cell and is used mainly as a solvent and as a dispersion medium for the colloidal system of protoplasm. Bound water, which accounts for only 4% of all cell water, is loosely connected to proteins by hydrogen bonds.
Due to the asymmetric charge distribution, the water molecule acts as a dipole and therefore can be bound by both positively and negatively charged protein groups. The dipole property of a water molecule explains its ability to orient itself in an electric field, to attach to various molecules and sections of molecules that carry a charge. This results in the formation of hydrates.
Due to its high heat capacity, water absorbs heat and thus prevents sudden temperature fluctuations in the cell. The water content in the body depends on its age and metabolic activity. It is highest in the embryo (90%) and gradually decreases with age. The water content of different tissues varies depending on their metabolic activity. For example, in the gray matter of the brain, water is up to 80%, and in the bones up to 20%. Water is the main means of moving substances in the body (blood flow, lymph, ascending and descending currents of solutions through the vessels of plants) and in the cell. Water serves as a "lubricant" material, necessary wherever there are rubbing surfaces (for example, in joints). Water has a maximum density at 4°C. Therefore, ice, which has a lower density, is lighter than water and floats on its surface, which protects the reservoir from freezing. This property of water saves the lives of many aquatic organisms.
1. What is the structure of water?
Answer. The water molecule has an angular structure: its constituent nuclei form an isosceles triangle, at the base of which are two hydrogens, and at the top is an oxygen atom. Internuclear O-N distances close to 0.1 nm, the distance between the nuclei of hydrogen atoms is 0.15 nm. Of the six electrons that make up the outer electronic layer oxygen atom in a water molecule, two electron pairs form covalent O-N connections, and the remaining four electrons are two unshared electron pairs.
The water molecule is a small dipole containing positive and negative charges at the poles. Near the hydrogen nuclei there is a lack of electron density, and on the opposite side of the molecule, near the oxygen nucleus, there is an excess of electron density. It is this structure that determines the polarity of the water molecule.
2. What is the amount of water (in%) contained in different cells?
The amount of water varies in different tissues and organs. So, in a person in the gray matter of the brain, its content is 85%, and in the bone tissue - 22%. The highest water content in the body is observed in the embryonic period (95%) and gradually decreases with age.
The water content in various plant organs varies within fairly wide limits. It varies depending on environmental conditions, age and type of plants. Thus, the water content in lettuce leaves is 93-95%, corn - 75-77%. The amount of water is not the same in different organs of plants: sunflower leaves contain 80-83% of water, stems - 87-89%, roots - 73-75%. The water content, equal to 6-11%, is typical mainly for air-dry seeds, in which vital processes are inhibited. Water is contained in living cells, in the dead elements of the xylem and in the intercellular spaces. In the intercellular spaces, water is in a vapor state. Leaves are the main evaporating organs of a plant. In this regard, it is natural that the largest amount of water fills the intercellular spaces of the leaves. In a liquid state, water is found in various parts of the cell: cell membrane, vacuole, cytoplasm. Vacuoles are the most water-rich part of the cell, where its content reaches 98%. At the highest water content, the water content in the cytoplasm is 95%. The lowest water content is characteristic of cell membranes. Quantitative determination of water content in cell membranes is difficult; apparently, it ranges from 30 to 50%. The forms of water in different parts of the plant cell are also different.
3. What is the role of water in living organisms?
Answer. Water is the predominant component of all living organisms. It has unique properties due to structural features: water molecules have the form of a dipole and hydrogen bonds form between them. The average water content in the cells of most living organisms is about 70%. Water in the cell is present in two forms: free (95% of all cell water) and bound (4-5% associated with proteins).
Water functions:
1. Water as a solvent. Many chemical reactions in the cell are ionic, so they only take place in an aquatic environment. Substances that dissolve in water are called hydrophilic (alcohols, sugars, aldehydes, amino acids), insoluble - hydrophobic (fatty acids, cellulose).
2. Water as a reagent. Water is involved in many chemical reactions: polymerization reactions, hydrolysis, in the process of photosynthesis.
3. Transport function. Movement through the body along with water of substances dissolved in it to its various parts and the removal of unnecessary products from the body.
4. Water as a heat stabilizer and thermostat. This function is due to such properties of water as high heat capacity - softens the impact on the body of significant temperature changes in the environment; high thermal conductivity - allows the body to maintain the same temperature throughout its volume; high heat of evaporation - used to cool the body during sweating in mammals and transpiration in plants.
5. Structural function. The cytoplasm of cells contains from 60 to 95% water, and it is she who gives the cells their normal form. In plants, water maintains turgor (the elasticity of the endoplasmic membrane), in some animals it serves as a hydrostatic skeleton (jellyfish)
Questions after § 7
1. What is the peculiarity of the structure of the water molecule?
Answer. The unique properties of water are determined by the structure of its molecule. The water molecule consists of an O atom bonded to two H atoms by polar covalent bonds. The characteristic arrangement of electrons in a water molecule gives it an electrical asymmetry. A more electronegative oxygen atom attracts the electrons of hydrogen atoms more strongly, as a result, the common pairs of electrons in the water molecule are shifted towards it. Therefore, although the water molecule is not charged as a whole, each of the two hydrogen atoms has a partially positive charge (denoted 8+), while the oxygen atom carries a partially negative charge (8-). The water molecule is polarized and is a dipole (has two poles).
The partially negative charge of the oxygen atom of one water molecule is attracted by the partially positive hydrogen atoms of other molecules. Thus, each water molecule tends to hydrogen bond with four neighboring water molecules.
2. What is the importance of water as a solvent?
Answer. Due to the polarity of molecules and the ability to form hydrogen bonds, water easily dissolves ionic compounds (salts, acids, bases). Well soluble in water and some non-ionic, but polar compounds, i.e., in the molecule of which there are charged (polar) groups, such as sugars, simple alcohols, amino acids. Substances that are highly soluble in water are called hydrophilic (from the Greek hygros - wet and philia - friendship, inclination). When a substance goes into solution, its molecules or ions can move more freely and, therefore, the reactivity of the substance increases. This explains why water is the main medium in which most chemical reactions take place, and all hydrolysis reactions and numerous redox reactions take place with the direct participation of water.
Substances that are poorly or completely insoluble in water are called hydrophobic (from the Greek phobos - fear). These include fats, nucleic acids, some proteins, and polysaccharides. Such substances can form interfaces with water, on which many chemical reactions take place. Therefore, the fact that water does not dissolve non-polar substances is also very important for living organisms. Among the physiologically important properties of water is its ability to dissolve gases (O2, CO2, etc.).
3. What is the thermal conductivity and heat capacity of water?
Answer. Water has a high heat capacity, i.e., the ability to absorb thermal energy with a minimal increase in its own temperature. The high heat capacity of water protects the tissues of the body from a rapid and strong increase in temperature. Many organisms cool themselves by evaporating water (transpiration in plants, sweating in animals).
4. Why consider that water is ideal fluid for a cell?
Answer. The high content of water in the cell is the most important condition for its activity. With the loss of most of the water, many organisms die, and a number of unicellular and even multicellular organisms temporarily lose all signs of life. This state is called suspended animation. After hydration, the cells wake up and become active again.
The water molecule is electrically neutral. But the electric charge inside the molecule is unevenly distributed: in the region of hydrogen atoms (more precisely, protons), positive charge, in the region where oxygen is located, the negative charge density is higher. Therefore, a particle of water is a dipole. The dipole property of a water molecule explains its ability to orient itself in an electric field, to attach itself to various molecules and sections of molecules that carry a charge. As a result, hydrates are formed. The ability of water to form hydrates is due to its universal dissolving properties. If the energy of attraction of water molecules to the molecules of a substance is greater than the energy of attraction between water molecules, then the substance dissolves. Depending on this, hydrophilic (Greek hydros - water and phileo - love) substances that are highly soluble in water (for example, salts, alkalis, acids, etc.) and hydrophobic (Greek hydros - water and phobos - fear) substances are distinguished, hardly or not at all soluble in water (fats, fat-like substances, rubber, etc.). The composition of cell membranes includes fat-like substances that limit the transition from the external environment to cells and vice versa, as well as from one part of the cell to another.
Most of the reactions that take place in a cell can only take place in an aqueous solution. Water is a direct participant in many reactions. For example, the breakdown of proteins, carbohydrates and other substances occurs as a result of their interaction with water catalyzed by enzymes. Such reactions are called hydrolysis reactions (Greek hydros - water and lysis - splitting).
Water has a high heat capacity and at the same time relatively high thermal conductivity for liquids. These properties make water an ideal liquid for maintaining the thermal balance of the cell and organism.
Water is the main environment for the flow of biochemical reactions of the cell. It is a source of oxygen released during photosynthesis, and hydrogen, which is used to restore assimilation products. carbon dioxide. And finally, water is the main means of transporting substances in the body (blood and lymph flow, ascending and descending currents of solutions through the vessels of plants) and in the cell.
5. What is the role of water in the cell
Ensuring cell elasticity. The consequences of the loss of water by the cell are wilting of leaves, drying of fruits;
Acceleration of chemical reactions due to the dissolution of substances in water;
Ensuring the movement of substances: the entry of most substances into the cell and their removal from the cell in the form of solutions;
Ensuring the dissolution of many chemicals (a number of salts, sugars);
Participation in a number of chemical reactions;
Participation in the process of thermoregulation due to the ability to slow heating and slow cooling.
6. What are the structural and physical Chemical properties water determine its biological role in the cell?
Answer. Structural physical and chemical properties of water determine its biological functions.
Water is a good solvent. Due to the polarity of molecules and the ability to form hydrogen bonds, water easily dissolves ionic compounds (salts, acids, bases).
Water has a high heat capacity, i.e., the ability to absorb thermal energy with a minimal increase in its own temperature. The high heat capacity of water protects the tissues of the body from a rapid and strong increase in temperature. Many organisms cool themselves by evaporating water (transpiration in plants, sweating in animals).
Water also has a high thermal conductivity, ensuring an even distribution of heat throughout the body. Consequently, the high specific heat capacity and high thermal conductivity make water an ideal liquid for maintaining the thermal balance of the cell and organism.
Water practically does not compress, creating turgor pressure, determining the volume and elasticity of cells and tissues. So, it is the hydrostatic skeleton that maintains the shape of roundworms, jellyfish and other organisms.
Water is characterized by the optimal value of the surface tension force for biological systems, which arises due to the formation of hydrogen bonds between water molecules and molecules of other substances. Due to the force of surface tension, capillary blood flow occurs, ascending and descending currents of solutions in plants.
In certain biochemical processes, water acts as a substrate.
Water is the most common chemical compound on Earth, its largest mass in a living organism. It is estimated that water makes up 85% of total weight average statistical cell. Whereas in human cells water is on average about 64%. However, the water content in different cells can vary significantly: from 10% in the cells of tooth enamel to 90% in the cells of the mammalian embryo. Moreover, young cells contain more water than old ones. So, in the cells of an infant, water is 86%, in the cells of an old person, only 50%.
In males, the water content in the cells is on average 63%, in females - slightly less than 52%. What caused it? It turns out that everything is simple. In the female body, there is a lot of adipose tissue, in the cells of which there is little water. Therefore, the water content in the female body is approximately 6-10% lower than in the male.
The unique properties of water are due to the structure of its molecule. From the course of chemistry, you know that the different electronegativity of hydrogen and oxygen atoms is the cause of the covalent polar bond in a water molecule. The water molecule has the shape of a triangle (87), in which electric charges located asymmetrically, and is a dipole (remember the definition of this term).
Due to the electrostatic attraction of the hydrogen atom of one water molecule to the oxygen atom of another molecule, hydrogen bonds arise between the water molecules.
The features of the structure and physico-chemical properties of water (the ability of water to be a universal solvent, variable density, high heat capacity, large surface tension, fluidity, capillarity, etc.), which determine its biological significance.
What functions does water perform in the body? Water is a solvent. The polar structure of the water molecule explains its properties as a solvent. Water molecules interact with chemicals, the elements of which have electrostatic bonds, and decompose them into anions and cations, which leads to chemical reactions. As you know, many chemical reactions occur only in aqueous solution. At the same time, the water itself remains inert, so it can be used in the body repeatedly. Water serves as a medium for transporting various substances within the body. In addition, the end products of metabolism are excreted from the body mainly in dissolved form.
There are two main types of solutions in living beings. (Remember the classification of solutions.)
The so-called true solution, when the molecules of the solvent are the same size as the molecules of the solute, they dissolve. As a result, dissociation occurs and ions are formed. In this case, the solution is homogeneous and, in scientific terms, consists of one liquid phase. Typical examples are solutions of mineral salts, acids or alkalis. Since there are charged particles in such solutions, they are able to conduct electricity and are electrolytes, like all solutions found in the body, including the blood of vertebrates, which contains many mineral salts.
A colloidal solution is the case when the solvent molecules are much smaller in size than the molecules of the solute. In such solutions, particles of a substance, which are called colloidal, move freely in the water column, since the force of their attraction does not exceed the force of their bonds with solvent molecules. Such a solution is considered heterogeneous, that is, consisting of two phases - liquid and solid. All biological fluids are mixtures, which include true and colloidal solutions, since they contain both mineral salts and huge molecules (for example, proteins) that have the properties of colloidal particles. Therefore, the cytoplasm of any cell, the blood or lymph of animals, and the milk of mammals simultaneously contain ions and colloidal particles.
As you probably remember, biological systems obey all the laws of physics and chemistry, therefore, in biological solutions, physical phenomena are observed that play a significant role in the life of organisms.
Water properties
Diffusion (from Latin Difusio - spreading, spreading, dispersion) in biological solutions manifests itself as a tendency to equalize the concentration of structural particles of dissolved substances (ions and colloidal particles), which ultimately leads to a uniform distribution of the substance in solution. It is thanks to diffusion that many unicellular creatures are fed, oxygen and nutrients are transported through the body of animals in the absence of circulatory and respiratory systems in them (remember what kind of animals they are). In addition, the transport of many substances to cells is carried out precisely due to diffusion.
Another physical phenomenon- osmosis (from the Greek. Osmosis - push, pressure) - the movement of the solvent through a semipermeable membrane. Osmosis causes the movement of water from a solution having a low concentration of solutes and a high content of H20 in a solution with a high concentration of solutes and a low water content. IN biological systems it is nothing but the transport of water at the cell level. That is why osmosis plays a significant role in many biological processes. The power of osmosis ensures the movement of water in plant and animal organisms, so that their cells receive nutrients and maintain a constant shape. It should be noted that the greater the difference in the concentration of a substance, the greater the osmotic pressure. Therefore, if the cells are placed in a hypotonic solution, they will swell and burst due to a sharp influx of water.
The vital activity of cells, tissues and organs of plants is due to the presence of water. Water is a constitutional substance. Determining the structure of the cytoplasm of cells and its organelles, due to the polarity of the molecules, it is a solvent for organic and inorganic compounds involved in metabolism, and acts as a background environment in which all biochemical processes take place. Easily penetrating through the shells and membranes of cells, water circulates freely throughout the plant, ensuring the transfer of substances and thus contributing to the unity of the metabolic processes of the body. Due to its high transparency, water does not interfere with the absorption of solar energy by chlorophyll.
The state of water in plant cells
Water in the cell is presented in several forms, they are fundamentally different. The main ones are constitutional, solvate, capillary and reserve water.
Some of the water molecules entering the cell form hydrogen bonds with a number of radical molecules organic matter. Hydrogen bonds are especially easy to form such radicals:
This form of water is called constitutional . It is contained by a cell with a strength of up to 90 thousand barr.
Due to the fact that water molecules are dipoles, they form solid aggregates with charged molecules of organic substances. Such water, associated with the molecules of organic substances of the cytoplasm by the forces of electrical attraction, is called solvate . Depending on the type of plant cell, the solvate water accounts for 4 to 50% of its total amount. Solvate water, like constitutional water, has no mobility and is not a solvent.
Much of the cell's water is capillary , because it is located in the cavities between macromolecules. Solvate and capillary water is held by the cell with a force called the matrix potential. It is equal to 15-150 bar.
Reserve called the water inside the vacuoles. The content of vacuoles is a solution of sugars, salts and a number of other substances. Therefore, the reserve water is retained by the cell with a force that is determined by the magnitude of the osmotic potential of the vacuolar content.
Water uptake by plant cells
Since there are no active carriers for water molecules in cells, its movement into and out of cells, as well as between neighboring cells, is carried out only according to the laws of diffusion. Therefore, solute concentration gradients turn out to be the main drivers for water molecules.
Plant cells, depending on their age and condition, absorb water using the sequential inclusion of three mechanisms: imbibition, solvation and osmosis.
imbibition . When seeds germinate, it begins to absorb water due to the imbibition mechanism. In this case, the vacant hydrogen bonds of the organic substances of the protoplast are filled, and water actively enters the cell from the environment. Compared to other forces operating in cells, the imbibition forces are colossal. For some hydrogen bonds, they reach a value of 90 thousand barr. At the same time, the seeds can swell and germinate in relatively dry soils. After all vacant hydrogen bonds are filled, imbibition stops and the following mechanism of water absorption is activated.
solvation . In the process of solvation, water absorption occurs by building hydration layers around the molecules of protoplast organic substances. The total water content of the cell continues to increase. The intensity of solvation essentially depends on the chemical composition of the protoplast. The more hydrophilic substances in the cell, the more fully the solvation forces are used. Hydrophilicity decreases in the series: proteins -> carbohydrates -> fats. Therefore, protein seeds (peas, beans, beans) absorb the largest amount of water per unit weight by solvation, starch seeds (wheat, rye) the intermediate one, and oilseeds (flax, sunflower) the smallest.
The solvation forces are inferior in power to the imbibition forces, but they are still quite significant and reach 100 bar. By the end of the solvation process, the water content of the cell is so great that capillary moisture settles down, and vacuoles begin to appear. However, from the moment of their formation, solvation stops, and further absorption of water is possible only due to the osmotic mechanism.
Osmosis . The osmotic mechanism of water uptake only works in cells that have a vacuole. The direction of water movement in this case is determined by the ratio of the osmotic potentials of the solutions included in the osmotic system.
The osmotic potential of the cell sap, denoted by R, is determined by the formula:
R = iRct,
where R - osmotic potential of cell sap
R- gas constant equal to 0.0821;
T - temperature on the Kelvin scale;
i- isotonic coefficient indicating the character electrolytic dissociation dissolved substances.
The isotonic ratio itself is equal to
And= 1 + α ( n + 1),
where α - degree of electrolytic dissociation;
P - the number of ions into which the molecule dissociates. For non-electrolytes P = 1.
The osmotic potential of a soil solution is usually denoted by the Greek letter π.
Water molecules always move from a medium with a lower osmotic potential to a medium with a higher osmotic potential. So, if the cell is in the soil (external) solution at R>π, then water enters the cells. The flow of water into the cell stops when the osmotic potentials are completely equalized (the vacuolar juice is diluted at the entrance of water absorption) or when the cell membrane reaches the limits of extensibility.
Thus, cells receive water from the environment only under one condition: the osmotic potential of the cell sap must be higher than the osmotic potential of the surrounding solution.
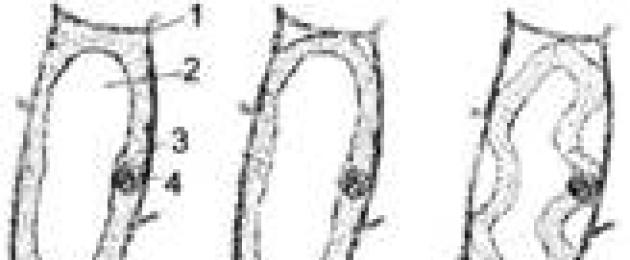
If R< π, there is an outflow of water from the cell into the external solution. In the course of fluid loss, the volume of the protoplast gradually decreases, it moves away from the membrane, and small cavities appear in the cell. Such a state is called Plasmolysis . The stages of plasmolysis are shown in fig. 3.18.
If the ratio of osmotic potentials corresponds to the condition P = π, then diffusion of water molecules does not occur at all.
A large amount of factual material indicates that the osmotic potential of the cell sap of plants varies within fairly wide limits. In agricultural plants, in root cells, it usually lies in an amplitude of 5-10 bar, in leaf cells it can rise up to 40 bar, and in fruit cells - up to 50 bar. In solonchak plants, the osmotic potential of cell sap reaches 100 bar.

Rice. 3.18.
A - a cell in a state of turgor; B - angular; B - concave; G - convex; D - convulsive; E - cap. 1 - shell; 2 - vacuole; 3 - cytoplasm; 4 - core; 5 - Hecht threads
1.3 Distribution of water in the cell
The water content in various plant organs varies within fairly wide limits. It varies depending on environmental conditions, age and type of plants. Thus, the water content in lettuce leaves is 93-95%, corn - 75-77%. The amount of water is not the same in different plant organs: sunflower leaves contain 80-83% of water, stems - 87-89%, roots - 73-75%. The water content, equal to 6-11%, is typical mainly for air-dry seeds, in which vital processes are inhibited.
Water is contained in living cells, in the dead elements of the xylem and in the intercellular spaces. In the intercellular spaces, water is in a vapor state. Leaves are the main evaporating organs of a plant. In this regard, it is natural that the largest amount of water fills the intercellular spaces of the leaves. In a liquid state, water is found in various parts of the cell: the cell membrane, vacuoles, and protoplasm. Vacuoles are the most water-rich part of the cell, where its content reaches 98%. At the highest water content, the water content in the protoplasm is 95%. The lowest water content is characteristic of cell membranes. Quantitative determination of water content in cell membranes is difficult; apparently, it ranges from 30 to 50%.
The forms of water in different parts of the plant cell are also different. The vacuolar cell sap is dominated by water retained by relatively low molecular weight compounds (osmotically bound) and free water. In the shell of a plant cell, water is mainly bound by high-polymer compounds (cellulose, hemicellulose, pectin substances), i.e., colloidally bound water. In the cytoplasm itself there is free water, colloidally and osmotically bound. Water located at a distance of up to 1 nm from the surface of a protein molecule is firmly bound and does not have a regular hexagonal structure (colloidal-bound water). In addition, there is a certain amount of ions in the protoplasm, and, consequently, part of the water is osmotically bound.
The physiological significance of free and bound water is different. Most researchers believe that the intensity of physiological processes, including growth rates, depends primarily on the content of free water. There is a direct correlation between the content of bound water and the resistance of plants to adverse external conditions. These physiological correlations are not always observed.
golgi apparatus
golgi apparatus
Lysosomes are small vesicles surrounded by a single membrane. They bud from the Golgi apparatus and possibly from the endoplasmic reticulum. Lysosomes contain a variety of enzymes that break down large molecules...
Health of schoolchildren: problems and solutions
When a teenager is engaged in sports, overtraining should not be allowed. Fatigue after a lot of physical activity is evidenced by lethargy, muscle pain. Parents should control the time of sports...
Information system cells
Genetic information is encoded in DNA. The genetic code was elucidated by M. Nirenberg and H.G. Quran, for which they were awarded the Nobel Prize in 1968. The genetic code is a system for the arrangement of nucleotides in nucleic acid molecules ...
Encoding and implementation of biological information in a cell, genetic code and its properties
Intermediary in the transfer genetic information(nucleotide order) from DNA to protein is mRNA (messenger RNA) ...
Meiobenthos of macrophyte thickets in the coastal zone of the Novorossiysk Bay
There are quite a lot of works describing the regularities of the spatial distribution of meiobenthic organisms - in recent decades this has been one of the most popular areas in research...
In 1890, Wilhelm Ostwald, who studied semi-permeable artificial films, suggested that semi-permeability could be the cause of not only osmosis, but also electrical phenomena. Osmosis occurs when...
Microbiology of fish and fish products
The microbiological assessment of water is given on the basis of the determination of the microbial number of QMAFAnM; if - titra; if - index; the presence of pathogenic microorganisms. The first two analyzes are ongoing...
Molecular genetic level of living structures
The fact that genes are located on chromosomes would seem to be inconsistent with the fact that humans have only 23 pairs of chromosomes and yet have thousands of different traits that thousands of different genes must match. Only signs...
Spherocerid flies (Diptera, Sphaeroceridae) of the nature reserve "Kamyshanova Polyana"
On the territory of the reserve "Kamyshanova Polyana" the following types of biotopes are clearly distinguished: forest, meadow, various near-water, as well as edge formations ...
Objects of biotechnology in the food industry
Metabolism, or metabolism, is the natural order of the transformation of substances and energy in living systems that underlies life, aimed at their conservation and self-reproduction; the totality of all chemical reactions that take place in the body ...
The concept of a cell
XVII century 1665 - English physicist R. Hooke in his work "Micrography" describes the structure of a cork, on thin sections of which he found correctly arranged voids. Hooke called these voids "pores, or cells" ...
The role of mitochondria in apoptosis
Physiology of cellular excitation
· The formation of cellular excitation is due precisely to the transport of ions. bilipid layer cell membrane impermeable to ions (Na, K, Cl), ion channels are designed for their transport into and out of the cell - special integral proteins ...
Chemical composition cells
All living organisms are capable of metabolism with environment. Processes are going on continuously in cells biological synthesis, or biosynthesis...
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0