The lesson is designed for 80–90 minutes. The topic of the lesson allows you to demonstrate to students the relationship of subjects such as biology, geography, chemistry, physics. In parentheses, there are options for answers to questions that I would like to receive from students.
Goals: familiarization of students with data on the content of water in cells of various tissues and water exchange in different organisms, with modern ideas about the structure and properties of water, its biological functions; improving the skills of logical thinking.
Equipment: physical map of the Earth, test tubes, glasses, capillary tubes; table salt, ethyl alcohol, sucrose, vegetable oil, paraffin, egg white, gastric juice, ice; reference books on physics and chemistry.
Organizing time
The teacher informs students about the topic and purpose of the lesson and the order of its conduct.
Knowledge check students on the topic "Elemental and chemical (molecular) composition of the cell." Three students work at the blackboard, the rest (according to options) work on cards.
Working at the blackboard
1. A list of elements is written on the board: F, Zn, N, Ca, J, Cl, Na, H, Mn, Cu, P, C, K, Fe, O, Mg, Co, from which you need to choose organogenic (biogenic) , macronutrients, microelements. Indicate their percentage in the cell.
(Student response: a) organogenic: N, H, C, O; b) macroelements: Ca, Cl, Na, Mn, P, K, Fe, Mg; c) trace elements: F, Zn, J, Cu, Co).
2. Give characteristics of organogenic elements. Explain why, during the development of life on Earth, these elements turned out to be "convenient" for the chemistry of life.
3. Write on the board information about the chemical (molecular) composition of the cell, indicating the percentage of the main classes of substances.
Work on cards
Answer the question in writing.
Option 1. How does the lack of any of the necessary elements (organogenic, macroelement, microelement) affect the vital activity of a cell, an organism? How can this be manifested? Give examples.
Option 2. What conclusion can be drawn from the fact that cells have a similar elemental and chemical (molecular) composition?
Option 3. What scientific significance is the data on the similarities and differences in the elemental composition (qualitative and quantitative) of living and inanimate nature?
Learning new material
Water content in cells and organisms
1. Read the poetic lines of Mikhail Dudnik and tell me if they are biologically correct. (The poem is written on the board.)
They say that eighty percent of water is a man,
From the water, I will add, his native rivers,
From the water, I will add, - the rains that made him drunk,
From water, I will add, from ancient water, springs.
From which his grandfathers and great-grandfathers drank ...
(Student response... The poetic lines are correct, because more than 2/3 of people consists of water.)
2. Looking at physical map, remember what is the ratio of the areas of land and the oceans on our planet.
(Student response... World Ocean, i.e. the water surrounding continents and islands occupies about 71% earth surface.)
Teacher comment... Water not only covers most the earth's surface, but also makes up the majority of all living things: microorganisms, plants, animals, humans.
3. Is water important in human life?
(Student response... A person drinks water, washes with it, uses it in various industries, in agriculture... Now many countries in the world lack fresh water, to obtain it, you have to build special plants, treatment facilities.)
Teacher comment... Water, such a familiar substance, has absolutely amazing properties. It was only thanks to these properties of water that life on Earth became possible. When looking for life on other planets, one of the most important questions is whether there is enough water there. The unique value of water for biological systems due to even simply its quantitative content in living organisms.
4. Give examples of the water content in cells of different organisms, their tissues and organs, known to you from courses in botany, zoology, human anatomy and physiology.
(Student response... Water makes up 80% of the cell mass in a young human or animal body and 60% in the cells of the old one. In the cells of the brain, it is 85%, and in the cells of the developing embryo - 90%. If a person loses 20% of water, then death occurs. True, not all human cells have such a high water content. For example, in the cells of the enamel of the teeth it is only 10-15%. There is a lot of water in the cells of the pulp of juicy fruits and leaves of plants, but there is very little of it in the cells of dry seeds or spores of plants and microorganisms, so they can be stored for a very long time until they are again watered under conditions conducive to their germination.)
5. What determines the differences in the water content in the cells?
(Student response... There is more water in those cells in which the metabolism is more intensive.)
Water intake into organisms of animals and plants
What methods do you know for obtaining water by different organisms?
(Student response... The pathways of water intake into the body are very diverse:
a) through the surface of the body - at unicellular organisms, lower plants, larvae of some insects, frogs, fish and other aquatic organisms;
b) with food and drink - in most animals;
c) there are animals that hardly drink or drink very little. This is possible due to: metabolic water, i.e. water formed in the body during oxidation, mainly of fats (when 1 g of fat is oxidized, 1.1 g of water is formed); economical use of water, which in some is ensured by the presence of waterproof covers, in others - by a high concentration of urine (for example, in camels, urine is 8 times more concentrated than plasma); water reserves (for example, in larvae);
d) plants absorb water from the soil using root hairs;
e) unusual ways of obtaining water are: epiphytes - plants that settle mainly on the trunks, branches of other trees - absorb water from the air; Many umbrella plants retain moisture in the cupped leaf sheaths, from where it is gradually absorbed through the epidermis.
Molecular structure and properties of water
Numerous biological functions carried out by water, are provided with its unique properties, and the uniqueness of the properties of water is determined by the structure of its molecule.
1. Remember the structural features of the water molecule known to you from the course of chemistry.
(Student response... In a water molecule (empirical formula H 2 O), one oxygen atom is covalently bonded to two hydrogen atoms. The molecule has the shape of a triangle, in one of the vertices of which there is an oxygen atom, and in the other two - a hydrogen atom.)
2. What is the nature of the covalent bond between the oxygen atom and hydrogen atoms?
(Student response... The bond between the oxygen atom and hydrogen atoms is polar, because oxygen attracts electrons more than hydrogen.)
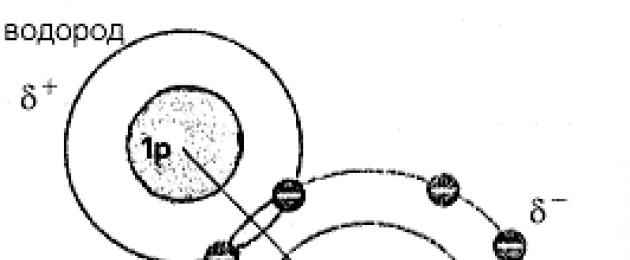
Teacher comment... Indeed, the oxygen atom, due to its greater electronegativity, attracts electrons more strongly than hydrogen atoms. The consequence of this is the polarity of the water molecule. In general, the water molecule is electrically neutral, but electric charge inside the molecule it is distributed unevenly, and in the region of hydrogen atoms a positive charge prevails, and in the region where oxygen is located - a negative charge (Fig. 1). Therefore, such a molecule is an electric dipole.

Rice. 1. A water molecule in which one oxygen atom is covalently bonded to two hydrogen atoms. The molecule is polar
The negatively charged oxygen atom of one water molecule attracts the positively charged hydrogen atoms of the other two molecules, so the water molecules are hydrogen bonded to each other. You are already familiar with the concept of a hydrogen bond (Fig. 2).

Rice. 2. Hydrogen bonds (lines) between water molecules; oxygen atoms (white circles) carry partial negative charges, so they form hydrogen bonds with hydrogen atoms (black circles) of other molecules that carry partial positive charges
In liquid water, these weak bonds are quickly formed and just as quickly destroyed by random collisions of molecules. It is thanks to the ability of water molecules to bind to each other using hydrogen bonds that water has a number of properties that are important for life.
Assignments for groups of students
The class is divided into five groups, each of which, using pre-prepared equipment, works according to the instruction card containing the task.
Task for the 1st group
You are offered a number of substances: table salt, ethyl alcohol, sucrose, vegetable oil, paraffin. Try to consistently dissolve these substances in water. Which of the proposed substances are soluble in water and which are not? Try to explain why some substances can dissolve in water, while others cannot. What property of water did you get acquainted with?
Assignment to the 2nd group
In a test tube with white flakes of insoluble egg white, heated in a water bath to 37 ° C, add gastric juice. What are you seeing? What reaction took place and thanks to what enzyme of gastric juice? What property of water have you met?
Assignment to the 3rd group
Dip the ice cubes into a glass of water. What are you seeing? What can you say about the density of water and ice? Specific information about the density of water and ice can be obtained from the "Handbook of Elementary Physics" (Enokhovich). What features of water have you met?
Assignment to the 4th group
You know that water boils and turns into a vaporous state at a temperature of 100 ° C. Using the Basic Physics Handbook, compare the boiling point of water with the boiling point of other liquids. Try to explain the results obtained.
Task for the 5th group
Try pouring water into a topped glass. Why is this possible? Slowly lower the small diameter glass tube into a glass of water. What are you seeing? Explain the results of the experiment. What property of water have you met?
1st group report
The proposed substances dissolve in water: table salt, ethyl alcohol, sucrose (cane sugar). Do not dissolve: vegetable oil and paraffin. From the results obtained, it can be concluded that substances with an ionic chemical bond (table salt), as well as non-ionic compounds (sugars, alcohols), in the molecules of which, probably, charged (polar) groups are present, dissolve in water. Water is one of the most versatile solvents: almost all substances dissolve in it, at least in trace amounts.
Teacher comment... If the energy of attraction between water molecules and molecules of a substance is greater than the energy of attraction between water molecules, then the substance dissolves. Water-soluble substances are called hydrophilic (salts, alkalis, acids, etc.). Non-polar (non-charge-bearing) compounds practically do not dissolve in water. They are called hydrophobic (fats, fat-like substances, rubber, etc.).
2nd group report
Insoluble egg white flakes dissolve under the action of pepsin of gastric juice. There is a reaction of enzymatic hydrolysis (cleavage) of proteins into amino acids with the addition of a water molecule at the rupture of each peptide bond... Similar reactions occur in the gastrointestinal tract of humans and animals:
Thus, water can enter into chemical reactions, i.e. is a reagent.
Water is the most abundant compound on Earth and in living organisms. The water content in cells depends on the nature of metabolic processes: the more intense they are, the higher the water content.
On average, an adult's cells contain 60-70% water. When 20% of water is lost, organisms die. A person can live no more than 7 days without water, while no more than 40 days without food.

Rice. 4.1. The spatial structure of a water molecule (H 2 O) and the formation of a hydrogen bond
The water molecule (H 2 O) consists of two hydrogen atoms that are covalently bonded to oxygen atoms. The molecule is polar, because it is bent at an angle and the nucleus of the oxygen atom pulls the shared electrons towards this angle, so that oxygen acquires a partial negative charge, and the hydrogen atoms at the open ends - partially positive charges. Water molecules are capable of being attracted to one another by a positive and negative charge, forming hydrogen bond (Figure 4.1.).
Due to the unique structure of water molecules and their ability to bind to each other using hydrogen bonds, water has a number of properties that determine it important role in the cell and the body.
Hydrogen bonds cause relatively high temperatures boiling and evaporation, high heat capacity and thermal conductivity of water, the property of a universal solvent.
Hydrogen bonds are 15-20 times weaker than covalent bonds. In the liquid state, hydrogen bonds are formed or broken, which causes the movement of water molecules, its fluidity.
The biological role of H 2 O
Water determines the physical properties of the cell - its volume, elasticity (turgor). The cell contains 95-96% free water and 4-5% bound water. Bound water forms water (solvation) shells around certain compounds (for example, proteins), preventing their interaction with each other.
Free water is a good solvent for many inorganic and organic polar substances. Substances that are readily soluble in water are called hydrophilic. For example, alcohols, acids, gases, most salts of Sodium, Potassium, etc. For hydrophilic substances, the binding energy between their atoms is less than the energy of attraction of these atoms to water molecules. Therefore, their molecules or ions are easily incorporated into the general system of water hydrogen bonds.
As a universal solvent, water plays an extremely important role as most chemical reactions occurs in aqueous solutions. The penetration of substances into the cell and the removal of waste products from it in most cases is possible only in a dissolved form.
Water does not dissolve non-polar (not carrying a charge) substances, since it cannot form hydrogen bonds with them. Water-insoluble substances are called hydrophobic ... These include fats, fat-like substances, polysaccharides, rubber.
Some organic molecules have dual properties: polar groups are located in some parts of them, and non-polar groups in others. Such substances are called amphipathic, or amphiphilic... These include proteins, fatty acids, phospholipids, nucleic acids. Amphiphilic compounds play an important role in the organization of biological membranes, complex supramolecular structures.
Water is directly involved in reactions hydrolysis- splitting of organic compounds. In this case, under the action of special enzymes to free valences organic molecules are joined by OH ions - and H + water. As a result, new substances with new properties are formed.
Water has a high heat capacity (i.e. the ability to absorb heat with insignificant changes in its own temperature) and good thermal conductivity. Due to these properties, the temperature inside the cell (and the body) is maintained at a certain level with significant changes in ambient temperature.
Important biological significance for the functioning of plants, cold-blooded animals has the fact that under the influence of dissolved substances (carbohydrates, glycerin) water can change its properties, in particular the freezing and boiling points.
The properties of water are so important for living organisms that it is impossible to imagine the existence of life as we know it, not only on Earth, but also on any other planet without a sufficient supply of water.
MINERAL SALTS
May be dissolved or undissolved. Molecules of mineral salts in an aqueous solution decompose into cations and anions.
1.3 Water distribution in the cage
The water content in various plant organs varies within fairly wide limits. It changes depending on environmental conditions, age and plant species. So, the water content in lettuce leaves is 93-95%, corn - 75-77%. The amount of water is not the same in different plant organs: in the leaves of sunflower water contains 80-83%, in the stems - 87-89%, in the roots - 73-75%. The water content, equal to 6-11%, is typical mainly for air-dry seeds, in which vital processes are inhibited.
Water is contained in living cells, in dead xylem elements and in intercellular spaces. In the intercellular spaces, water is in a vaporous state. The main evaporating organs of the plant are the leaves. In this regard, it is natural that the greatest amount of water fills the intercellular spaces of the leaves. In a liquid state, water is found in various parts of the cell: cell membrane, vacuole, protoplasm. Vacuoles are the richest part of the cell with water, where its content reaches 98%. With the highest water content, the water content in the protoplasm is 95%. The lowest water content is characteristic of the cell membranes. It is difficult to quantify the water content in cell membranes; apparently, it ranges from 30 to 50%.
Forms of water in different parts plant cell are also different. Vacuolar cell sap is dominated by water held by relatively low molecular weight compounds (osmotically bound) and free water. In the shell of a plant cell, water is mainly bound by high-polymer compounds (cellulose, hemicellulose, pectin substances), i.e., colloid-bound water. In the cytoplasm itself there is free water, colloid and osmotically bound. Water located at a distance of up to 1 nm from the surface of the protein molecule is firmly bound and does not have a regular hexagonal structure (colloid-bound water). In addition, there is a certain amount of ions in the protoplasm, and therefore, part of the water is osmotically bound.
The physiological significance of free and bound water is different. Most researchers believe that the intensity of physiological processes, including growth rates, depends primarily on the content of free water. There is a direct correlation between the content of bound water and the resistance of plants to unfavorable external conditions. These physiological correlations are not always observed.
Golgi apparatus
Golgi apparatus
Lysosomes are small vesicles surrounded by a single membrane. They bud off from the Golgi apparatus and possibly from the endoplasmic reticulum. Lysosomes contain a variety of enzymes that break down large molecules ...
Schoolchildren's health: problems and solutions
When a teenager is engaged in sports, overtraining should not be allowed. Fatigue after intense physical exertion is indicated by lethargy, muscle pain. Parents should control the time of sports ...
Information system cells
Genetic information is encoded in DNA. The genetic code was discovered by M. Nirenberg and H.G. Quran, for which they were awarded the Nobel Prize in 1968. Genetic code is a system of arrangement of nucleotides in nucleic acid molecules ...
Coding and implementation of biological information in a cell, genetic code and its properties
Transmission intermediary genetic information(nucleotide order) mRNA (messenger RNA) comes from DNA to protein ...
Meiobenthos of macrophyte thickets of the coastal zone of the Novorossiysk Bay
There are a lot of works describing the regularities of the spatial distribution of meiobenthos organisms - in recent decades it has been one of the most popular directions in research ...
In 1890, Wilhelm Ostwald, who worked on semi-permeable artificial films, suggested that semi-permeability could be the cause not only of osmosis, but also of electrical phenomena. Osmosis occurs then ...
Microbiology of fish and fish products
Microbiological assessment of water is given on the basis of determining the microbial number KMAFAnM; koli - titer; koli - index; the presence of pathogenic microorganisms. The first two analyzes are carried out continuously ...
Molecular genetic level of living structures
The fact that genes are located on chromosomes, it would seem, does not correspond to the fact that humans have only 23 pairs of chromosomes and at the same time thousands of different traits, which must correspond to thousands of different genes. Signs alone ...
Spherocerid flies (Diptera, Sphaeroceridae) of the Kamyshanova Polyana nature reserve
On the territory of the Kamyshanova Polyana reserve, the following types of biotopes are clearly distinguished: forest, meadow, various near-water, as well as edge formations ...
Biotechnology objects in the food industry
Metabolism, or metabolism, is the underlying natural order of transformation of substances and energy in living systems, aimed at their preservation and self-reproduction; the totality of all chemical reactions in the body ...
Cell concept
XVII century 1665 - English physicist R. Hooke in his work "Micrography" describes the structure of the cork, on the thin sections of which he found correctly located voids. Hooke called these voids "pores, or cells" ...
Role of mitochondria in apoptosis
Physiology of cellular excitation
· The formation of cellular excitement is due precisely to the transport of ions. Bilipid layer cell membrane impermeable to ions (Na, K, Cl), ion channels are intended for their transport into and out of the cell - special integral proteins ...
Chemical composition cells
All living organisms are capable of metabolism with environment... Processes are ongoing in the cells biological synthesis, or biosynthesis ...
The water content in various plant organs varies within fairly wide limits. It changes depending on environmental conditions, age and plant species. So, the water content in lettuce leaves is 93-95%, corn - 75-77%. The amount of water is not the same in different plant organs: in the leaves of sunflower water contains 80-83%, in the stems - 87-89%, in the roots - 73-75%. The water content, equal to 6-11%, is typical mainly for air-dry seeds, in which vital processes are inhibited.
Water is contained in living cells, in dead xylem elements and in intercellular spaces. In the intercellular spaces, water is in a vaporous state. The main evaporating organs of the plant are the leaves. In this regard, it is natural that the greatest amount of water fills the intercellular spaces of the leaves. In a liquid state, water is found in various parts of the cell: the cell membrane, vacuoles, cytoplasm. Vacuoles are the richest part of the cell with water, where its content reaches 98%. With the highest water content, the water content in the cytoplasm is 95%. The lowest water content is characteristic of the cell membranes. It is difficult to quantify the water content in cell membranes; apparently, it ranges from 30 to 50%.
The forms of water in different parts of the plant cell are also different. Vacuolar cell juice is dominated by water held by relatively low molecular weight compounds (osmotically bound) and free water. In the shell of a plant cell, water is mainly bound by high-polymer compounds (cellulose, hemicellulose, pectin substances), i.e., colloid-bound water. In the cytoplasm itself there is free water, colloid and osmotic bound. Water located at a distance of up to 1 nm from the surface of the protein molecule is firmly bound and does not have a regular hexagonal structure (colloid-bound water). In addition, there is a certain amount of ions in the cytoplasm, and, therefore, part of the water is osmotically bound.
The physiological significance of free and bound water is different. According to most researchers, the intensity of physiological processes, including growth rates, depends primarily on the content of free water. There is a direct correlation between the content of bound water and the resistance of plants to unfavorable external conditions. These physiological correlations are not always observed.
For their normal existence, cells and the plant organism as a whole must contain a certain amount of water. However, this is easily accomplished only for plants growing in water. For land plants, this task is complicated by the fact that water in the plant organism is continuously lost during the evaporation process. The evaporation of water by a plant reaches enormous proportions. An example can be given: one corn plant evaporates up to 180 kg of water during the growing season, and 1 hectare of forest in South America evaporates on average 75 thousand kg of water per day. The huge consumption of water is due to the fact that most plants have a significant leaf surface located in an atmosphere not saturated with water vapor. At the same time, the development of an extensive leaf surface is necessary and developed in the course of a long evolution to ensure a normal supply of carbon dioxide contained in the air in an insignificant concentration (0.03%). In his famous book "Fighting Plants with Drought" K.A. Timiryazev pointed out that the contradiction between the need to capture carbon dioxide and to reduce the consumption of water left an imprint on the structure of the entire plant organism.
In order to compensate for the loss of water during evaporation, a large amount of it must continuously enter the plant. Two continuous processes in the plant - the intake and evaporation of water - are called water balance of plants. For normal growth and development of plants, it is necessary that the water flow approximately corresponds to the arrival, or, in other words, that the plant reduces its water balance without a large deficit. For this, in the plant, in the process of natural selection, adaptations have been developed to absorb water (a colossally developed root system), to the movement of water (a special conducting system), to reduce evaporation (a system of integumentary tissues and a system of automatically closing stomatal openings).
Despite all these adaptations, a water deficit is often observed in a plant, that is, the flow of water is not balanced by its consumption in the process of transpiration.
Physiological disorders occur in different plants with varying degrees of water deficiency. There are plants that have developed in the process of evolution a variety of adaptations to the transfer of dehydration (drought-resistant plants). The elucidation of the physiological characteristics that determine the resistance of plants to a lack of water is the most important problem, the solution of which is of great not only theoretical, but also agricultural practical importance. At the same time, in order to solve it, it is necessary to know all aspects of water exchange in a plant organism.
V earth crust occurs about 100 chemical elements, but only 16 of them are needed for life. The most common in plant organisms are four elements - hydrogen, carbon, oxygen, nitrogen, which form various substances. The main components of a plant cell are water, organic and mineral substances.
Water- the basis of life. The water content in plant cells ranges from 90 to 10%. It is a unique substance due to its chemical and physical properties... Water is necessary for the process of photosynthesis, transport of substances, cell growth, it is a medium for many biochemical reactions, a universal solvent, etc.
Minerals (ash)- substances that remain after burning a piece of any organ. The ash content ranges from 1% to 12% dry weight. Almost all the elements that make up water and soil are found in the plant. The most common are potassium, calcium, magnesium, iron, silicon, sulfur, phosphorus, nitrogen (macronutrients) and copper, aluminum, chlorine, molybdenum, boron, zinc, lithium, gold (trace elements). Mineral substances play an important role in the life of cells - they are part of amino acids, enzymes, ATP, electron transport chains, they are necessary to stabilize membranes, participate in metabolic processes, etc.
Organic matter plant cells are subdivided into: 1) carbohydrates, 2) proteins, 3) lipids, 4) nucleic acids, 5) vitamins, 6) phytohormones, 7) products of secondary metabolism.
Carbohydrates make up up to 90% of the substances that make up the plant cell. Distinguish:
Monosaccharides (glucose, fructose). Monosaccharides are formed in leaves during photosynthesis and are easily converted into starch. They accumulate in fruits, less often in stems and bulbs. Monosaccharides are transported from cell to cell. They are energetic material and participate in the formation of glycosides.
Disaccharides (sucrose, maltose, lactose, etc.) are formed from two particles of monosaccharides. They accumulate in roots and fruits.
Polysaccharides are polymers that are very widespread in plant cells. This group of substances includes starch, inulin, cellulose, hemicellulose, pectin substances, and callose.
Starch is the main reserve substance of the plant cell. Primary starch is formed in chloroplasts. In the green parts of the plant, it breaks down to mono- and disacchares and is transported along the phloem of the veins to the growing parts of the plant and the storage organs. In the leukoplasts of storage organs, secondary starch is synthesized from sucrose in the form of starch grains.
The starch molecule is composed of amylose and amylopectin. Linear amylose chains, consisting of several thousand glucose residues, are able to branch spirally and thus take on a more compact form. In the branched amylopectin polysaccharide, compactness is ensured by intensive branching of chains due to the formation of 1,6-glycosidic bonds. Amylopectin contains approximately twice as many glucose residues as amylose.
With Lugol's solution, an aqueous suspension of amylose gives a dark blue color, a suspension of amylopectin - red-violet, a suspension of starch - blue-violet.
Inulin is a polymer of fructose, a storage carbohydrate of the Asteraceae family. It is in the cells in a dissolved form. Does not stain with iodine solution, stained with β-naphthol red.
Cellulose is a polymer of glucose. Cellulose contains about 50% of the carbon found in the plant. This polysaccharide is the main material of the cell wall. Cellulose molecules are long chains of glucose residues. A plurality of OH groups protrude from each chain. These groups are directed in all directions and form hydrogen bonds with adjacent chains, which ensures rigid cross-linking of all chains. The chains are united with each other, forming microfibrils, and the latter are combined into larger structures - macrofibrils. The tensile strength with this structure is very high. Macrofibrils, arranged in layers, are immersed in a cementing matrix consisting of pectin substances and hemicelluloses.
Cellulose does not dissolve in water; with a solution of iodine it gives a yellow coloration.
Pectins are composed of galactose and galacturonic acid. Pectic acid is a polygalacturonic acid. They are part of the matrix of the cell wall and provide its elasticity. Pectins form the basis of the median lamina that forms between cells after division. Form gels.
Hemicelluloses are high-molecular compounds of mixed composition. They are part of the cell wall matrix. They do not dissolve in water, they hydrolyze in an acidic environment.
Callose is an amorphous glucose polymer found in various parts of the plant organism. Callose is formed in the sieve tubes of the phloem, and is also synthesized in response to damage or adverse effects.
Agar-agar is a high molecular weight polysaccharide contained in seaweed... It dissolves in hot water, and solidifies after cooling.
Squirrels high molecular weight compounds consisting of amino acids. Elemental composition - C, O, N, S, P.
Plants are able to synthesize all amino acids from simpler substances. The 20 essential amino acids form the entire variety of proteins.
The complexity of the structure of proteins and the extreme diversity of their functions make it difficult to create a unified clear classification of proteins on any one basis. By composition, proteins are classified into simple and complex. Simple - consist only of amino acids, complex - consist of amino acids and non-protein material (prosthetic group).
Simple proteins include albumins, globulins, histones, prolamins, gluteins. Albumin - neutral proteins, soluble in water, rarely found in plants. Globulins are neutral proteins, insoluble in water, soluble in dilute saline solutions, common in seeds, roots, and plant stems. Histones are neutral proteins, soluble in water, localized in the nuclei of all living cells. Prolamins - soluble in 60-80% ethanol, are found in cereals. Gluteins are soluble in alkaline solutions and are found in caryopses of cereals, green parts of plants.
The complex includes phosphoproteins (prosthetic group - phosphoric acid), lycoproteins (carbohydrate), nucleoproteins (nucleic acid), chromoproteins (pigment), lipoproteins (lipid), flavoproteins (FAD), metalloproteins (metal).
Proteins play an important role in the life of a plant organism and, depending on the function performed, proteins are subdivided into structural proteins, enzymes, transport proteins, contractile proteins, storage proteins.
Lipids- organic substances insoluble in water and soluble in organic solvents (ether, chloroform, benzene). Lipids are divided into true fats and lipoids.
True fats are esters of fatty acids and some alcohol. They form an emulsion in water; when heated with alkalis, they hydrolyze. They are reserve substances, accumulate in seeds.
Lipoids are fatty substances. These include phospholipids (part of membranes), wax (form a protective coating on leaves and fruits), sterols (part of protoplasm, participate in the formation of secondary metabolites), carotenoids (red and yellow pigments, necessary to protect chlorophyll, give color fruits, flowers), chlorophyll (the main pigment of photosynthesis)
Nucleic acids- the genetic material of all living organisms. Nucleic acids (DNA and RNA) are made up of monomers - nucleotides. A nucleotide molecule is composed of a five-carbon sugar, a nitrogenous base, and phosphoric acid.
Vitamins- complex organic substances of various chemical composition. They have high physiological activity - they are necessary for the synthesis of proteins, fats, for the work of enzymes, etc. Vitamins are subdivided into fat-soluble and water-soluble. Fat-soluble vitamins include vitamins A, K, E, water-soluble vitamins C, B vitamins.
Phytohormones- low molecular weight substances with high physiological activity. They have a regulatory effect on the growth and development of plants at very low concentrations. Phytohormones are divided into stimulants (cytokinins, auxins, gibberellins) and inhibitors (ethylene and abscisines).
- In contact with 0
- Google+ 0
- OK 0
- Facebook 0